INVESTOR PRESENTATION JANUARY 2021
Published on January 11, 2021
Exhibit 99.1

1 NASDAQ: AEMD Investor Presentation January 2021

2 This investor presentation contains forward - looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995, including, without limitation: the ability to enroll patients in the Early Feasibility Studies; the ability to successfully complete the Early Feasibility Studies and achieve the endpoints for the studies, or any future studies with the Hemopurifier or to successfully develop and commercialize the Hemopurifier; the ability to demonstrate the removal of exosomes with the Hemopurifier; the potential synergistic use of the Hemopurifier with chemotherapy, immunotherapy and targeted agents; the ability to demonstrate the removal of SARS - CoV - 2/COVID - 19 glycoproteins with the Hemopurifier; the potential initiation of a SARS - CoV - 2 clinical trial; the ability to establish collaborations and to raise capital; and financial strength and guidance. These statements are subject to a number of risks, uncertainties and assumptions, including, but not limited to: the risks associated with Covid - 19 and other pandemic risks; the timing and success of Aethlon’s studies and trials; our ability to enroll patients in our studies and trials on a timely basis, or at all; the Early Feasibility Studies and potential safety and other complications thereof; the ability to obtain regulatory approval within the timeframe expected, or at all; complications associated with product development and commercialization activities; the scope, progress and expansion of developing Aethlon’s product candidates; the size and growth of the market(s) therefor and the rate and degree of market acceptance thereof vis - à - vis alternative therapies; and Aethlon’s ability to attract or retain key management, members of the board of directors and personnel. In light of these risks and uncertainties, and other risks and uncertainties that are described in the Risk Factors section of Aethlon’s Form 10 - Q filed with the SEC on October 28, 2020, subsequent 10 - Q filings, and other filings that Aethlon makes with the SEC from time to time (which are available at http://www.sec.gov), the events and circumstances discussed in such forward - looking statements may not occur, and Aethlon’s actual results could differ materially and adversely from those anticipated or implied thereby. Any forward - looking statements speak only as of the date of this presentation and are based on information available to Aethlon as of the date of this presentation. FORWARD LOOKING STATEMENTS

3 The Aethlon Hemopurifier ® ▪ Two FDA “Breakthrough Device” designations • Safety in over 150 patient treatments in human trials or emergency use • Proprietary mechanism of action • Clears life - threatening glycosylated viruses • Designed to clear cancer promoting exosomes

4 Aethlon Medical ඵ +HPRSXULILHU LQKXPDQWULDOVIRURQFRORJ\DQGYLUDO GLVHDVHV ඵ ([SHULHQFHGPDQDJHPHQWWHDP ඵ 6WURQJFDVKSRVLWLRQ ඵ 1RQ GLOXWLYH1&,IXQGLQJIRUPXOWLSOHSURJUDPV ඵ %DVHGLQ6DQ 'LHJR
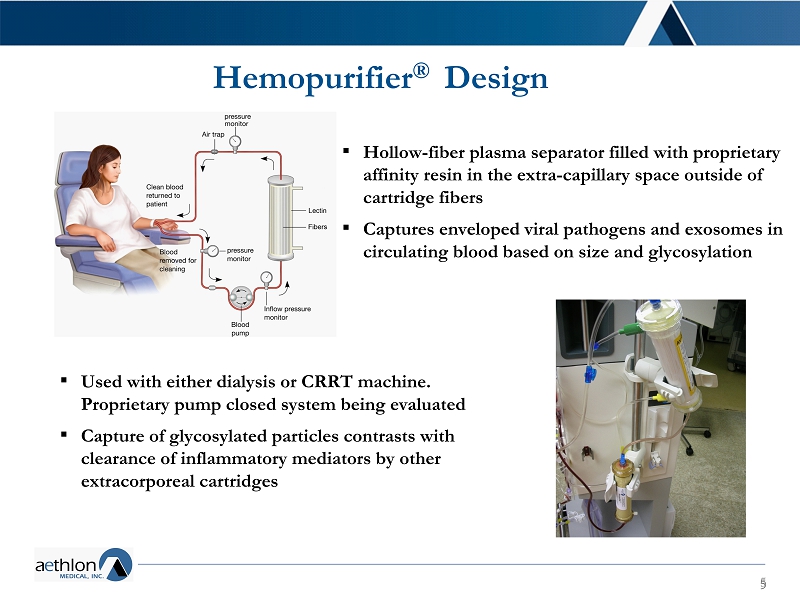
5 5 ▪ Hollow - fiber plasma separator filled with proprietary affinity resin in the extra - capillary space outside of cartridge fibers ▪ Captures enveloped viral pathogens and exosomes in circulating blood based on size and glycosylation Hemopurifier ® Design ▪ Used with either dialysis or CRRT machine. Proprietary pump closed system being evaluated ▪ Capture of glycosylated particles contrasts with clearance of inflammatory mediators by other extracorporeal cartridges
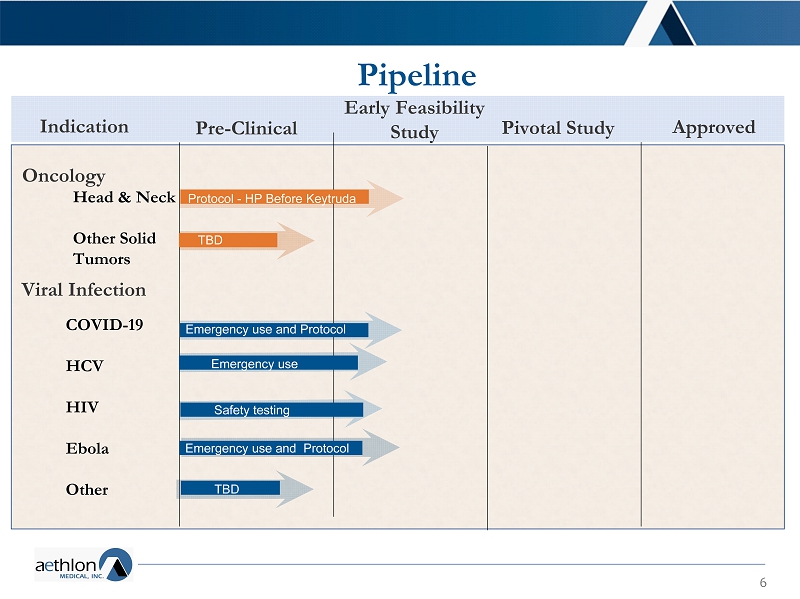
6 Pipeline Pre - Clinical (DUO\)HDVLELOLW\ 6WXG\ Pivotal Study Approved Indication Oncology Viral Infection Head & Neck Other Solid Tumors COVID - 19 HCV HIV Ebola Other 6DIHW\WHVWLQJ Emergency use and Protocol Emergency use Emergency use and Protocol Protocol - HP Before Keytruda TBD TBD

7 +HPRSXULILHU 3URJUDPV ▪ Clearance of cancer promoting exosomes ▪ Potentially synergistic with chemotherapy, immunotherapy, targeted agents ▪ Multiple potential clinical targets: • Breast, head and neck, gastrointestinal, melanoma, other solid tumors ▪ Well characterized markets, development pathways & endpoints ▪ Early feasibility study (EFS) in head and neck cancer initiated with first patient treatment at University of Pittsburgh Medical Center in December 2020 Oncology

8 ▪ Key mediators in cell communication and potent drivers in healing and repair ▪ They shed from both normal and malignant cells ▪ Primary means of intra - cellular communication ▪ Tumor derived exosomes (TEX) — released by tumor cells that promote: • Metastasis, chemotherapy/targeted therapy resistance, immune suppression ▪ There is no existing treatment for depleting tumor - derived exosomes ▪ Also involved in viral disease – inflammation, coagulopathy ▪ Hemopurifier ® is the first candidate capable of clearing exosomes Why Exosomes?
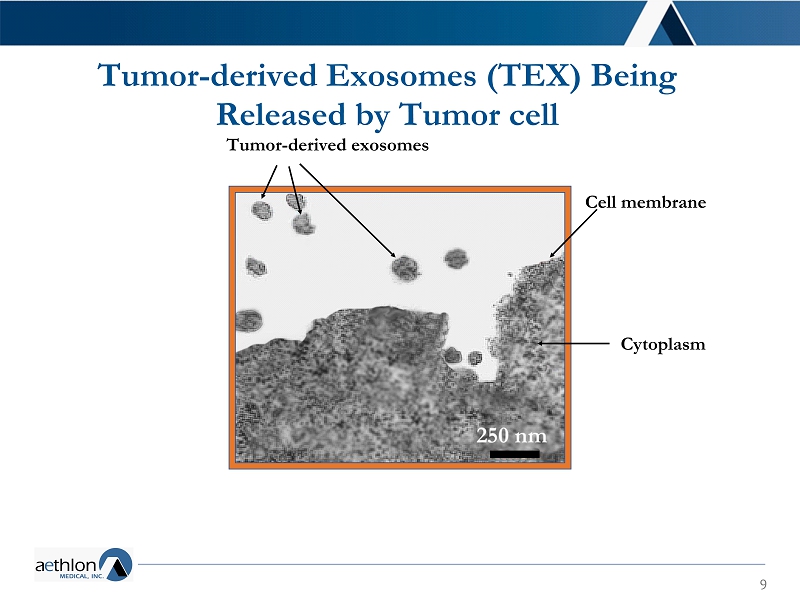
9 Tumor - derived Exosomes (TEX) Being Released by Tumor cell Tumor - derived exosomes &HOOPHPEUDQH Cytoplasm

1 0 ▪ Phase I contract from NCI — Completed “Device Strategy for Differential Isolation of Oncosomes and Non - Malignant Exosomes” ▪ NCI SBIR Grant — Completed “The Hemopurifier ® Device for Targeted Removal of Breast Cancer Exosomes from the Blood Circulation” ▪ Phase II NCI SBIR Contract — September 2019 • $1.8 million over 2 years “Technologies for Differential Isolation of Exosomes and Oncosomes ” ▪ NIDCR RO1 — July 2020 • Collaboration with University of Pittsburgh, MGH, UHawaii • $3.5 million over 5 years “Depleting exosomes to improve responses to immune therapy in head and neck squamous cell carcinoma” National Cancer Institute Studies

1 1 ▪ NCT #04453046 ▪ University of Pittsburgh Hillman Cancer Center ▪ 10 - 12 subjects with advanced or metastatic HNSSC ▪ Combination with pembrolizumab (Keytruda ® ) ▪ Keytruda approved June 2019 in front line setting ▪ 4 - hour Hemopurifier treatment immediately prior to Keytruda ▪ Endpoints: Safety, exosome clearance and characterization • ORR, PFS, OS ▪ First patient treated in December 2020 EFS in Head and Neck Cancer
1 2 Hemopurifier ® Treatment of Viral Infections ඵ 'HPRQVWUDWHGFOHDUDQFHRIPXOWLSOHGLIIHUHQWYLUXVHV LQYLWUR +,9GHQJXH:HVW1LOHLQIOXHQ]D(ERODKHUSHV0(56 ඵ 6DIHW\DQGYLUDOFOHDUDQFHLQIRXUKXPDQFOLQLFDOWULDOVLQ+&9 ඵ 2YHU VXFFHVVIXODSSOLFDWLRQVLQKXPDQVZLWK+&9ZLWKQRVDIHW\LVVXHV ඵ 6LQJOHSDWLHQWWUHDWPHQWVLQ(ERODDQG+,9 2SHQSURWRFROIRUHPHUJHQF\8VHLQ86DQG&DQDGDIRU(EROD ,'(6XSSOHPHQWIRU&29,' -XQH
1 3 Hemopurifier ® Treatment of SARS - CoV - 2/COVID - 19 ▪ Circulating virus correlates with cytokine levels and poor outcome ▪ Hemopurifier has been shown to clear MERS, another coronavirus ▪ Clears SARS - CoV - 2/COVID - 19 glycoproteins based on in vitro experiments ▪ IDE supplement for COVID - 19 approved June 17, 2020 ▪ New Feasibility Study starting ▪ Approved for 20 sites — 40 patients in total ▪ ICU patients with severe or life - threatening symptoms and central IV access ▪ Patient already treated under Single Patient Emergency Use regulations

1 4 Charles J. Fisher, Jr., M.D., FACP, FCCP, FCCM, Chief Executive Officer ▪ Academic & Industry thought leader in sepsis & inflammation ▪ Head of critical care — Cleveland Clinic ▪ 35 years industry development experience ▪ Senior executive — Lilly, Abbott, Cardiome James B. Frakes, MBA, Senior VP & Chief Financial Officer ▪ 29 years public company CFO experience ▪ Investment banking & venture capital Steven LaRosa, MD, Chief Medical Officer (CMO) • 25 Years Infectious Diseases, Critical Care, Coagulation, Inflammation, Infectious Diseases, Cancer Guy Cipriani, MBA, Senior VP & Chief Business Officer • 20 Years experience in public companies as CBO Thomas L. Taccini , VP Manufacturing & Product Development ▪ Over 35 years experience in engineering ▪ Product development and quality systems Aethlon Medical Senior Management Team

1 5 Strong Cash Position ▪ September 30, 2020 Company’s cash balance was approximately $14.5 million ▪ No debt ▪ NASDAQ: AEMD ~12.1 million shares outstanding

1 6 8SFRPLQJ1HZV $HWKORQ+HPRSXULILHU ඵ ,'(VXSSOHPHQWDSSURYHGIRU6$56 &R9 &29,' ඵ (PHUJHQF\WUHDWPHQWRI6$56 &R9 &29,' SDWLHQWV ඵ ()6LQLWLDWHGIRUKHDGDQGQHFNFDQFHUDQGILUVWSDWLHQW WUHDWHG ඵ ([SHFWHG1HZV ඵ ,QLWLDWLRQRI6$56 &R9 WULDO ඵ (DUO\FOLQLFDOGDWDIURP()6WULDOV ඵ 3URRIRIFRQFHSWIRURWKHUVROLGWXPRUV

1 7 Summary ▪ Unique Hemopurifier ® blood purification device ▪ Two FDA Breakthrough Designations ▪ Multiple therapeutic targets in cancer and viral disease ▪ Management team with well over 100 years healthcare experience

1 8 This presentation may contain predictions, estimates, and other forward looking statements that involve risks and uncertainti es, including whether and when our products are successfully developed and introduced; market acceptance of the Aethlon Hemopurifier ® and other product offerings; regulatory delays, manufacturing delays, and other risks detailed in our SEC filings, which are accessible at www.sec.gov or on our website: www.AethlonMedical.com 9635 Granite Ridge Drive, Suite 100 San Diego, California 92123 858.459.7800 Nasdaq: AEMD www.AethlonMedical.com