INVESTOR PRESENTATION
Published on October 30, 2018
Exhibit 99.1

Image of Ebola viruses exiting host cells - Courtesy of NIAID NYC O NCOLOGY C ONFERENCE N ASDAQ : AEMD - M ARKET CAP : ~$20 M J IM J OYCE - F OUNDER & CEO O CTOBER 30, 2018 1

FORWARD LOOKING STATEMENTS The following presentation may contain predictions, estimates, and other forward looking statements that involve risks and uncertainties, including whether and when our products are successfully developed and introduced ; market acceptance of the Aethlon Hemopurifier® and other product offerings ; regulatory delays, manufacturing delays, and other risks detailed in our SEC filings, which are accessible at www . sec . gov or on our website : www . AethlonMedical . com 2

T O A DDRESS U NMET N EEDS IN G LOBAL H EALTH F OCUS 3

T O S AVE L IVES M ISSION 4

A FIRST - IN - CLASS THERAPEUTIC TECHNOLOGY T HE H EMOPURIFIER ® D ESIGNED FOR THE SINGLE - USE DEPLETION OF CIRCULATING VIRUSES AND CANCER - PROMOTING EXOSOMES O UR L EAD THERAPEUTIC C ANDIDATE 5

D EPLOYED FOR USE ON THE GLOBAL INFRASTRUCTURE OF DIALYSIS & CRRT INSTRUMENTS T HE H EMOPURIFIER ® D ESIGNED FOR THE SINGLE - USE DEPLETION OF CIRCULATING VIRUSES AND CANCER - PROMOTING EXOSOMES 6
The Aethlon Hemopurifier® M ECHANISM OF ACTION F ULL CIRCULATION DEPLETION OF VIRUSES AND CANCER PROMOTING EXOSOMES TARGETS ARE GLYCAN SHIELDED TO EVADE THE HOST IMMUNE RESPONSE C OMBINATION M ECHANISM OF ACTION P LASMA SEPARATION / LECTIN AFFINITY CAPTURE 7
The Aethlon Hemopurifier® V IRAL C LINICAL ACCOMPLISHMENTS I N VITRO C APTURE VALIDATIONS OF 15 HIGH - THREAT VIRAL PATHOGENS C OMPLETED F OUR INVESTIGATIONAL HUMAN STUDIES OUTSIDE U.S. C ONCLUDED U.S. HUMAN FEASIBILITY STUDY (M ARCH 2017) R ECEIVED “ EXPEDITED A CCESS P ATHWAY ” (EAP) D ESIGNATION FROM FDA (S EPTEMBER 2017) 8

T HE H EMOPURIFIER ® IS AN FDA DESIGNATED “B REAKTHROUGH D EVICE ” FOR THE TRE A TMENT OF LIFE - THREATENING G L YCOSYL A TED VIRUSES T H A T ARE NOT ADDRESSED WITH APPROVED THERAPIES . T HE A ETHLON H EMOPURIFIER ® Designated a “Breakthrough Device” by FDA 9
Ebola Image Courtesy of NIAID E BOLA VIRUS A CASE STUDY IN TREATING A LIFE - THREATENING VIRUS NOT ADDRESSED WITH AN APPROVED THERAPY 10

Frankfurt University Hospital APPRO V AL E MERGENCY - USE FROM G ERMANY ' S F EDERAL I NSTITUTE FOR D RUGS AND M EDICAL D EVICES (B F A R M) TO ADMINISTER H EMOPURIFIER ® THERAPY TO AN E BOLA - INFECTED PHYSICIAN AT F RANKFURT U NIVERSITY H OSPITAL . 11

T HE TREATMENT OF E BOLA VIRUS A SINGLE 6.5 - HOUR ADMINISTRATION THERAPY W AS OF H EMOPURIFIER ® DELIVERED TO THE P A TIEN T , WHO WITH MU L TIPLE W AS COM A T OSE ORGAN FAILURE . 12

E BOLA T REATMENT R ESULTS P RESENTED AT THE A MERICAN S OCIETY OF N EPHROLOGY A NNUAL M EETING BY H ELMUT G EIGER , M.D., C HIEF OF N EPHROLOGY AT F RANKFURT U NIVERSITY H OSPITAL • H EMOPURIFIER ® THERAPY WAS WELL TOLERATED WITH NO ADVERSE EVENTS • P RE - TREATMENT VIRAL LOAD PRIOR WAS MEASURED TO BE 400,000 COPIES / ML • P OST - TREATMENT VIRAL LOAD WAS MEASURED AT 1,000 COPIES / ML • P ATIENT MADE A FULL RECOVERY 13

T HE TREATMENT OF E BOLA VIRUS D R . S TEFAN B ÜTTNER HOLDING THE H EMOPURIFIER ® AFTER TREATMENT OF E BOLA VIRUS 14

V IRUS C APTURE A SSAY R ESULT 253 MILLION COPIES OF E BOLA VIRUS CAPTURED WITHIN THE HEMOPURIFIER ® DURING THE 6.5 HOUR TREATMENT Analysis: BSL4 Lab Philipps University Marburg (O. Dolnik/M. Eickmann/S. Becker) 15

T HE H EMOPURIFIER ® TO TREAT CANCER ? 16

A DDRESSING A SIGNIFICANT UNMET NEED IN CANCER CARE M ISSION 17
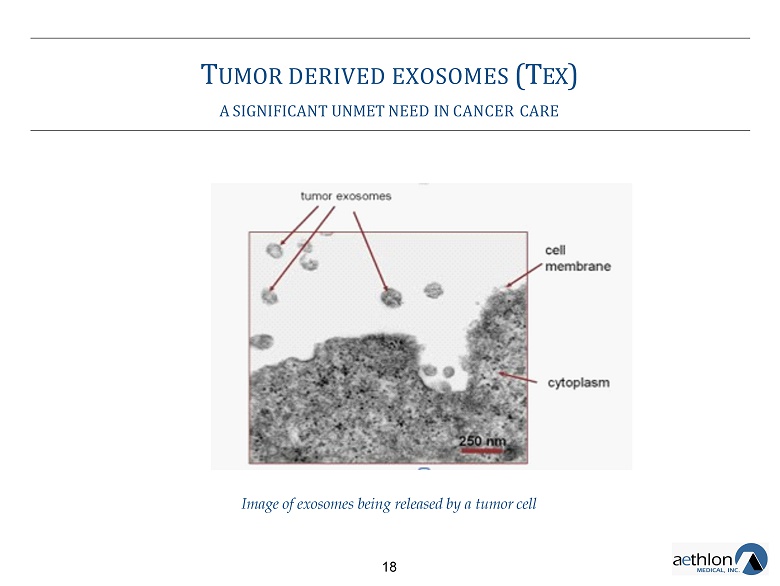
T UMOR DERIVED EXOSOMES (T EX ) A SIGNIFICANT UNMET NEED IN CANCER CARE Image of exosomes being released by a tumor cell 18
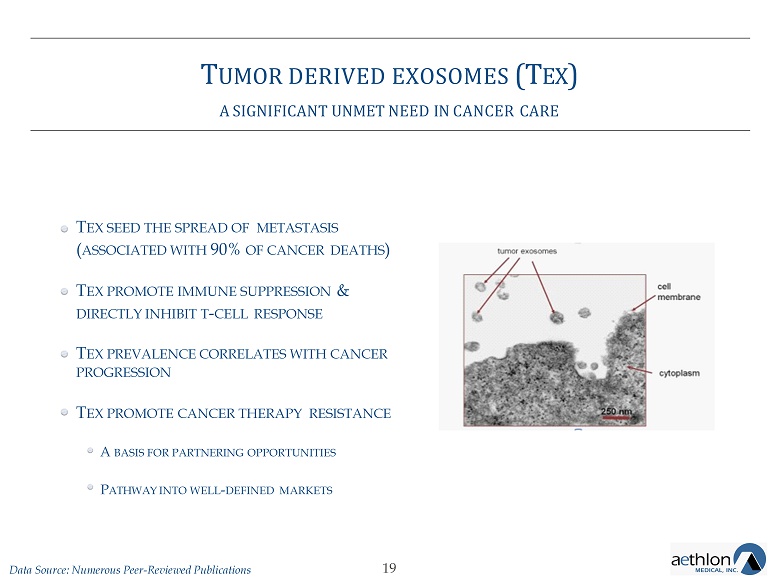
T UMOR DERIVED EXOSOMES (T EX ) A SIGNIFICANT UNMET NEED IN CANCER CARE T EX SEED THE SPREAD OF METASTASIS ( ASSOCIATED WITH 90% OF CANCER DEATHS ) T EX PROMOTE IMMUNE SUPPRESSION & DIRECTLY INHIBIT T - CELL RESPONSE T EX PREVALENCE CORRELATES WITH CANCER PROGRESSION T EX PROMOTE CANCER THERAPY RESISTANCE A BASIS FOR PARTNERING OPPORTUNITIES P ATHWAY INTO WELL - DEFINED MARKETS 19 Data Source: Numerous Peer - Reviewed Publications
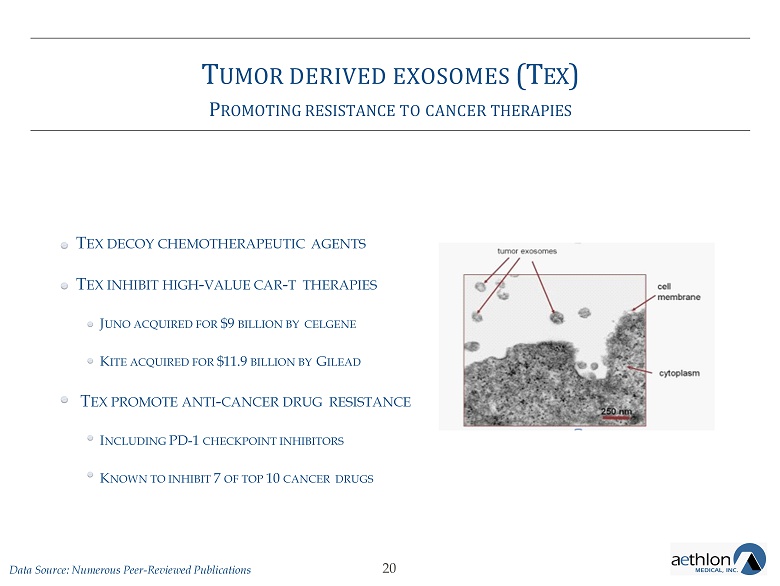
T UMOR DERIVED EXOSOMES (T EX ) P ROMOTING RESISTANCE TO CANCER THERAPIES T EX DECOY CHEMOTHERAPEUTIC AGENTS T EX INHIBIT HIGH - VALUE CAR - T THERAPIES J UNO ACQUIRED FOR $9 BILLION BY CELGENE K ITE ACQUIRED FOR $11.9 BILLION BY G ILEAD T EX PROMOTE ANTI - CANCER DRUG RESISTANCE I NCLUDING PD - 1 CHECKPOINT INHIBITORS K NOWN TO INHIBIT 7 OF TOP 10 CANCER DRUGS 20 Data Source: Numerous Peer - Reviewed Publications
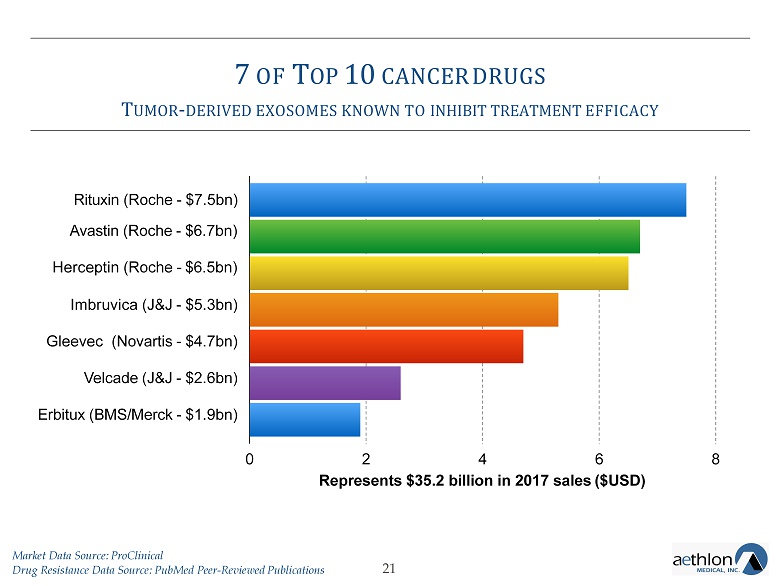
7 OF T OP 10 CANCER DRUGS T UMOR - DERIVED EXOSOMES KNOWN TO INHIBIT TREATMENT EFFICACY 21 Rituxin (Roche - $7.5bn) Avastin (Roche - $6.7bn) Herceptin (Roche - $6.5bn) Imbruvica (J&J - $5.3bn) Gleevec (Novartis - $4.7bn) Velcade (J&J - $2.6bn) Erbitux (BMS/Merck - $1.9bn) 0 2 4 6 Represents $35.2 billion in 2017 sales ($USD) 8 Market Data Source: ProClinical Drug Resistance Data Source: PubMed Peer - Reviewed Publications
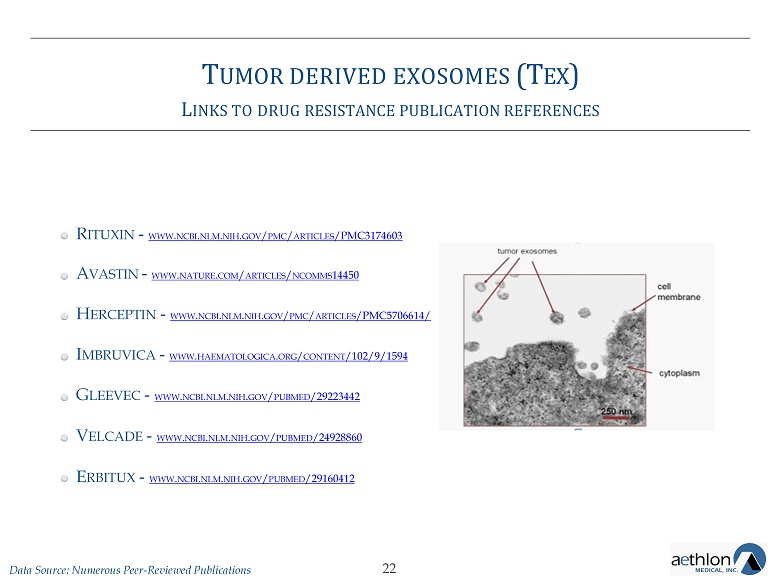
T UMOR DERIVED EXOSOMES (T EX ) L INKS TO DRUG RESISTANCE PUBLICATION REFERENCES R ITUXIN - WWW . NCBI . NLM . NIH . GOV / PMC / ARTICLES /PMC3174603 A VASTIN - WWW . NATURE . COM / ARTICLES / NCOMMS 14450 H ERCEPTIN - WWW . NCBI . NLM . NIH . GOV / PMC / ARTICLES /PMC5706614/ I MBRUVICA - WWW . HAEMATOLOGICA . ORG / CONTENT /102/9/1594 G LEEVEC - WWW . NCBI . NLM . NIH . GOV / PUBMED /29223442 V ELCADE - WWW . NCBI . NLM . NIH . GOV / PUBMED /24928860 E RBITUX - WWW . NCBI . NLM . NIH . GOV / PUBMED /29160412 22 Data Source: Numerous Peer - Reviewed Publications

INTEREST IN THE H EMOPURIFIER TO TREAT CANCER F OLLOWING PUBLICATION HAS BEEN CITED 183 TIMES 23 J Transl Med. 2012 Jun 27;10:134. doi: 10.1186/1479 - 5876 - 10 - 134 . EXOSOME REMOVAL AS A THERAPEUTIC ADJUVANT IN CANCER Marleau AM 1, Chen CS , Joyce JA , Tullis RH . Author information 1 : Aethlon Medical Inc, 8910 University Center Lane, Suite 660 , San Diego, CA 92122 , USA . annette@aethlonmedical . com Abstract Exosome secretion is a notable feature of malignancy owing to the roles of these nanoparticles in cancer growth, immune suppression, tumor angiogenesis and therapeutic resistance . Exosomes are 30 - 100 nm membrane vesicles released by many cells types during normal physiological processes . Tumors aberrantly secrete large quantities of exosomes that transport oncoproteins and immune suppressive molecules to support tumor growth and metastasis . The role of exosomes in intercellular signaling is exemplified by human epidermal growth factor receptor type 2 (HER 2 ) over - expressing breast cancer, where exosomes with the HER 2 oncoprotein stimulate tumor growth and interfere with the activity of the therapeutic antibody Herceptin® . Since numerous observations from experimental model systems point toward an important clinical impact of exosomes in cancer, several pharmacological strategies have been proposed for targeting their malignant activities . We also propose a novel device strategy involving extracorporeal hemofiltration of exosomes from the entire circulatory system using an affinity plasmapheresis platform known as the Aethlon ADAPT™ (adaptive dialysis - like affinity platform technology) system, which would overcome the risks of toxicity and drug interactions posed by pharmacological approaches . This technology allows affinity agents, including exosome - binding lectins and antibodies, to be immobilized in the outer - capillary space of plasma filtration membranes that integrate into existing kidney dialysis systems . Device therapies that evolve from this platform allow rapid extracorporeal capture and selective retention of target particles < 200 nm from the entire circulatory system . This strategy is supported by clinical experience in hepatitis C virus - infected patients using an ADAPT™ device, the Hemopurifier®, to reduce the systemic load of virions having similar sizes and glycosylated surfaces as cancer exosomes . This review discusses the possible therapeutic approaches for targeting immune suppressive exosomes in cancer patients, and the anticipated significance of these strategies for reversing immune dysfunction and improving responses to standard of care treatments . Citation Source: Google Scholar as of 9/14/18 Publication Source: Journal of Translational Medicine

2018 C ANCER I NITIATIVES M ISSION 24

NIH/NCI Contract 2018 I NITIATIVE #1 “D EVICE S TRATEGY FOR SELECTIVE ISOLATION OF ONCOSOMES AND NON - MALIGNANT EXOSOMES ” C OMPLETED J UNE 2018 D EMONSTRATED ISOLATION AND CAPTURE OF METASTATIC MELANOMA EXOSOMES P REPARING P HASE II CONTRACT SUBMISSION ( VALUED AT $2 MILLION ) 25

NIH/NCI Contract 2018 I NITIATIVE #2 “T HE H EMOPURIFIER DEVICE FOR TARGETED REMOVAL OF BREAST CANCER EXOSOMES FROM THE BLOOD CIRCULATION ” CONTRACT AWARD ON S EPTEMBER 18, 2018 PROGRAM INITIATED 26

UCI Clinical Study 2018 I NITIATIVE #3 “P LASMA EXOSOME CONCENTRATION IN CANCER PATIENTS UNDERGOING TREATMENT ” M ULTI - INDICATION ENROLLMENT OF CANCER PATIENTS D EMONSTRATED ( MAY - AUGUST 2018) IN VITRO CAPTURE OF EXOSOMES UNDERLYING : B REAST CANCER E SOPHAGEAL CANCER O VARIAN CANCER 27

NIH/NCI SUBMISSION 2018 I NITIATIVE #4 H UMAN CLINICAL STUDY PROPOSAL ENTITLED : “ DEPLETING EXOSOMES TO IMPROVE RESPONSES TO IMMUNE THERAPY IN HEAD AND NECK CANCER SQUAMOUS CELL CANCER ” A DJUNCT STUDY WITH O PDIVO C URRENTLY P ENDING REVIEW PANEL DECISION 28
FDA Breakthrough Device Submission 2018 I NITIATIVE #5 “U SE OF THE A ETHLON H EMOPURIFIER AS AN ADJUNCT THERAPY IN PATIENTS WITH DIAGNOSED METASTATIC CANCER ” B REAKTHROUGH SUBMISSION ON S EPTEMBER 25, 2018 P ENDING DECISION FROM FDA 29

DOD/U.S. Army Breakthrough Award 2018 I NITIATIVE #6 “I SOLATION OF TRIPLE NEGATIVE BREAST CANCER E XOSOMES USING THE H EMOPURIFIER ” D EPARTMENT OF DEFENSE ( DOD ) CONGRESSIONALLY DIRECTED MEDICAL RESEARCH PROGRAM (CDMRP), BREAST CANCER BREAKTHROUGH RESEARCH AWARD A WARD NOTIFICATION ON O CTOBER 23, 2018 P ENDING COMPLETION OF CONTRACTING PROCESS 30

A FIRST - IN - CLASS THERAPEUTIC TECHNOLOGY T HE H EMOPURIFIER ® D ESIGNED FOR THE SINGLE - USE DEPLETION OF CIRCULATING VIRUSES AND CANCER PROMOTING EXOSOMES O UR L EAD THERAPEUTIC C ANDIDATE 31

T O A DDRESS U NMET N EEDS IN G LOBAL H EALTH F OCUS 32

T O S AVE L IVES M ISSION 33

This presentation may contain predictions, estimates, and other forward looking statements that involve risks and uncertainties, including whether and when our products are successfully developed and introduced ; market acceptance of the Aethlon Hemopurifier® and other product offerings ; regulatory delays, manufacturing delays, and other risks detailed in our SEC filings, which are accessible at www . sec . gov or on our website : www . AethlonMedical . com 9635 Granite Ridge Drive, Suite 100 San Diego, California 92123 858.459.7800 Nasdaq: AEMD ww w .AethlonMedical. c om 34