ROTH INVESTOR CONFERENCE PRESENTATION
Published on March 14, 2017
Exhibit 99.1

A e thlon Medical, Inc . Nasdaq: AEMD ROTH INVESTOR CONFERENCE MARCH 14, 2017 Jim Joyce Chairman, CEO
| 1 |

FORWARD LOOKING STATEMENTS The following presentation may contain predictions, estimates, and other forward looking statements that involve risks and uncertainties, including whether and when our products are successfully developed and introduced ; market acceptance of the A e thlon Hemopurifier ® and other product offerings ; regulatory delays, manufacturing delays, and other risks detailed in our SEC filings, which are accessible at www . sec . gov or on our website : www . A e thlonMedical . com
| 2 |

THERAPEUTIC TECHNOLOGIES TO ADDRESS UNMET NEEDS IN GLOBAL HEALTH & BIODEFENSE
| 3 |

SAVE LIVES
| 4 |

CORPORATE FOCUS • The Treatment of Infectious Viral Pathogens • Candidate Pipeline – Cancer – Neurological Disorders • Exosome Sciences Subsidiary – Focused on the discovery of companion biomarkers that underlie Aethlon therapeutic targets
| 5 |

The A e thlon Hemopurifier® A First - In - Class Broad - Spectrum Technology Designed For The Single - Use Removal of Viruses From Blood
| 6 |

DEPLOYED WITHIN THE GLOBAL INFRASTRUCTURE OF DIALYSIS & CRRT MACHINES
| 7 |

The A e thlon Hemopurifier® x Immediate virus elimination x Prior to cell & organ infection x Inhibits progeny virus replication x Companion assay quantifies v irus capture x Broad - spectrum mechanism
| 8 |

Clinical Development History • Initiated & Completed x >20 in vitro validation studies x Four investigational human studies (outside U.S.) x ~150 human treatment experiences Hepatitis C virus (HCV), HIV and Ebola virus (EBV) x FDA feasibility study on March 13, 2017
| 9 |

About the Feasibility Study • A Clinical Safety Study in End - Stage Renal Disease (ESRD) Subjects Infected with Hepatitis C Virus (HCV) • DaVita Med Center Dialysis – Houston, TX. • Primary Objective x To demonstrate the safety of the Aethlon Hemopurifier® x No device - related adverse events reported • Secondary Objective – To quantify the number of viruses captured during treatment – To measure changes in viral load before and after treatment • Data being assembled for final FDA report • Estimated Study Enrollment: 10 Subjects – Concluded at 8 subjects
| 10 |

Forthcoming FDA Submissions • Submit Feasibility Study Final Report • File Pre - Submission Document To Request Follow - on Guidance Underlying – Market clearance pathways for virulent viruses that do not permit for controlled human studies – Study design for viral targets for which controlled human studies are feasible – The 21 st Century Cures Act (became law in December) • Mandates a priority review program for medical devices that target diseases for which no FDA - approved alternatives are available
| 11 |

The Global Health Threat of Viral Pathogens A Significant Therapeutic Void • 300+ viruses known to be infectious to man – Only a fraction are addressed with an antiviral drug • Only 1 of 13 Category “A” viruses are addressed – Easily transmitted, high fatality rate threats likely to disrupt the normal functioning of society • No solution for antiviral drug resistance • No solution for the natural emergence of new viruses • No solution for genetically engineered viruses of mass destruction
| 12 |

“A genetically engineered virus could kill more people than nuclear weapons — and yet no country on Earth is ready for the threat . ” Bill Gates Munich Security Conference February 18, 2017
| 13 |

To fulfill the broad - spectrum medical countermeasure objective of the U . S . Department of Health and Human Services (HHS) 2016 Public Health Emergency Medical Countermeasure Enterprise (PHEMCE) . This initiative is directed toward bioterror, pandemic threats and other pathogens that are not well addressed with drug or vaccine therapies . Strategic Goal
| 14 |

“ The only strategy to address the breadth of viruses that could emerge naturally through mother nature or be created by man as agents of bioterror” Ken Alibek Keynote Speech at Crossing Boundaries International Symposium on Bioterrorism Former Director of the Soviet Union’s Biological Weapon Program Author of BIOHAZARD The A e thlon Hemopurifier®
| 15 |
The A e thlon Hemopurifier® Opportunities in Viral Indications with an Approved Antiviral Drug x Adjunct to improve benefit of antiviral drug agents x Antiviral drug resistance
| 16 |

The A e thlon Hemopurifier® Opportunities in Viruses not Addressed with an Antiviral Drug x Known pandemic viral t hreats x Naturally emerging viruses x Agents of Bioterrorism Including genetically engineered viruses
| 17 |

I n vitro capture study validations
| 18 |
Chronic Viral Threats • Hemopurifier Capture Validations x Human Immunodeficiency Virus (HIV) x Hepatitis C Virus (HCV) • Approved Antiviral Drug (U.S.) – HIV antivirals suppress disease progression – HCV antivirals are often curative □ Drug resistance remains a significant issue
| 19 |
Reactivated Latent Viral Threats • Hemopurifier Capture Validations x Cytomegalovirus (CMV) x Epstein - Barr Virus (EBV) x Herpes Simplex Virus - 1 (HSV - 1) • Approved Antiviral Drug (U.S. ) – CMV ( Yes), EBV (No), HSV (Yes) □ Significant need for broad - spectrum solution to address immune - suppression related virus reactivation □ Opportunity in Sepsis, Organ Transplants
| 20 |

Bioterror/Pandemic Threats • Hemopurifier Capture Validations x Ebola Virus x Lassa Virus x Middle - East Respiratory Syndrome (MERS - CoV) x Smallpox (Based on Monkeypox & Vaccinia Models) • Approved Antiviral Drug (U.S.) – None – Smallpox vaccine in the U.S. National Stockpile
| 21 |
Pandemic Influenza Threats • Hemopurifier Capture Validations x H1N1 Swine Flu Virus x H5N1 Bird Flu Virus x Spanish Flu of 1918 Virus (reconstructed) • Approved Antiviral Drug (U.S.) – None for highly virulent influenza strains
| 22 |

Mosquito - Born Pandemic Threats • Hemopurifier Capture Validations x Chikungunya Virus x Dengue Virus x West Nile Virus x Zika Virus • Approved Antiviral Drug (U.S.) – None
| 23 |

Proof of Concept Against a Highly Virulent Virus
| 24 |

The Treatment of Ebola Virus Frankfurt University Hospital Special approval from The Federal Institute for Drugs and Medical Devices (BfArM)
| 25 |
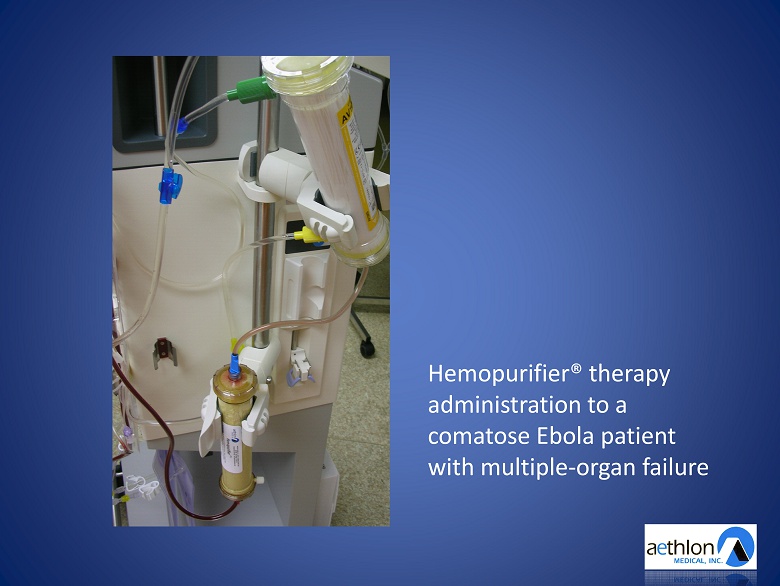
Hemopurifier® therapy administration to a comatose Ebola patient with multiple - organ failure
| 26 |

Dr. Stefan Büttner Holding Hemopurifier® After Ebola Treatment
| 27 |

Ebola Treatment Data Presented By Dr. Helmut Geiger American Society of Nephrology Annual Meeting • 6.5 hour Hemopurifier® therapy administration • Pre - treatment viral load: 400,000 copies/ml • Post - treatment viral load: 1,000 copies/ml • Patient made full recovery • Companion assay validated capture of 258M viruses – Hemopurifier® since approved in United States & Canada under Emergency - Use provisions
| 28 |

“Top 25 Best Inventions” “11 Most Remarkable Advances in Healthcare”
| 29 |
The A e thlon Hemopurifier® A Potential Role in Other Disease Conditions
| 30 |

Candidate Pipeline Targeting Disease Promoting Exosomes • Cancer – Tumor - derived exosomes promote spread of metastasis and immune suppression x Capture of Breast, Ovarian and Melanoma Validated • Tauopathies – A class of 20+ neurodegenerative disorders – Hallmark is abnormal aggregation of tau protein in brain – Exosomes discovered to be a tau transport mechanism – Exploratory phase • Alzheimer’s Disease, Chronic Traumatic Encephalopathy (CTE)
| 31 |

| 32 |
Discovery of TauSome™ A Candidate Tauopathy Biomarker
| 33 |

A BIOMARKER TO DIAGNOSE CHRONIC TRAUMATIC ENCEPHALOPATHY (CTE) IN THE LIVING?
| 34 |

• TauSome testing was conducted in the DETECT study – In collaboration with the Boston University CTE Center – First NIH funded CTE research study – 78 former NFL players (high risk CTE group) – 16 athlete controls (low risk CTE group)
| 35 |

Preliminary Study of Plasma Exosomal Tau as a Potential Biomarker for Chronic Traumatic Encephalopathy Stern , Tripodis, Baugh, Fritts, Martin, Chaisson, Cantu, Joyce, Shah, Ikezu, Zhang, Gercel - Taylor, & Taylor J Alzheimer’s Disease, 2016 • Findings suggest that TauSome plasma levels may be an accurate, noninvasive CTE biomarker – TauSome levels significantly higher in the NFL group – TauSome levels correlated with cognitive decline
| 36 |
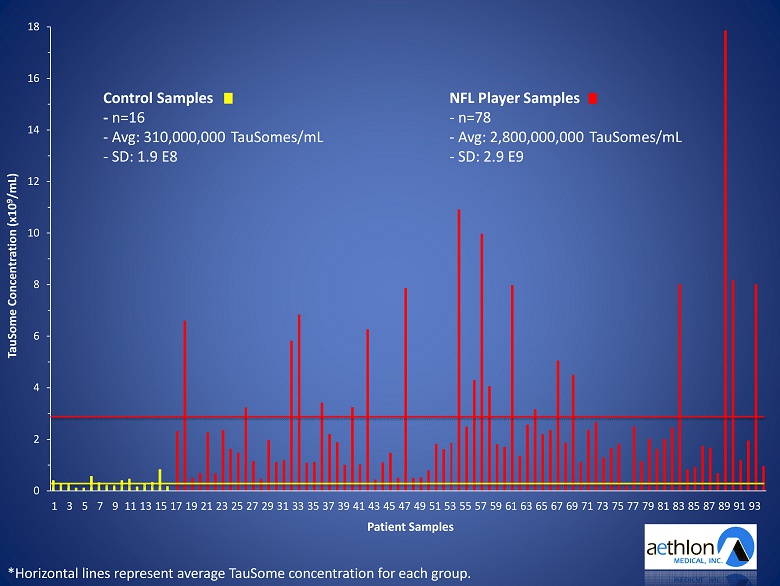
0 2 4 6 8 10 12 14 16 18 1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39 41 43 45 47 49 51 53 55 57 59 61 63 65 67 69 71 73 75 77 79 81 83 85 87 89 91 93 TauSome Concentration (x10 9 /mL) Patient Samples *Horizontal lines represent average TauSome concentration for each group. Control Samples - n=16 - Avg: 310,000,000 TauSomes/mL - SD: 1.9 E8 NFL Player Samples - n=78 - Avg: 2,800,000,000 TauSomes/mL - SD: 2.9 E9
| 37 |

WE ALSO HAD THE OPPORTUNITY TO EXPLORE TAUSOME™ LEVELS IN ALZHEIMERS’S PATIENTS
| 38 |
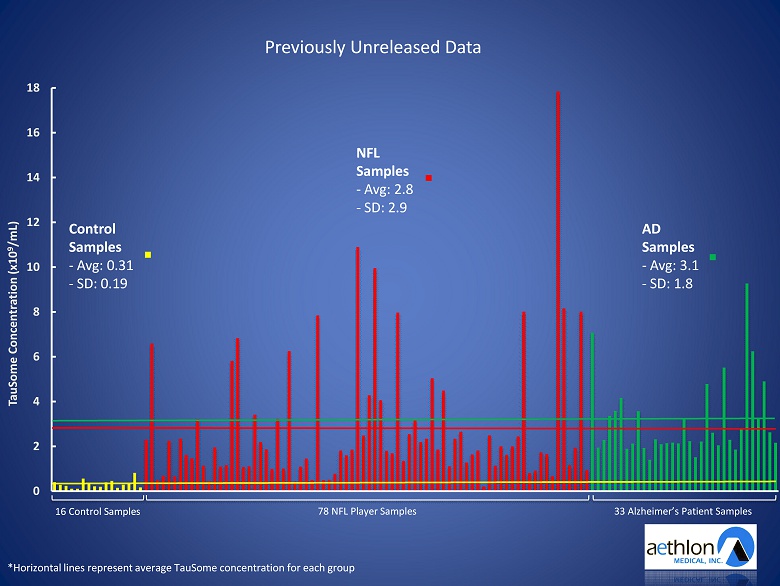
0 2 4 6 8 10 12 14 16 18 TauSome Concentration (x10 9 /mL) *Horizontal lines represent average TauSome concentration for each group 16 Control Samples 78 NFL Player Samples 33 Alzheimer’s Patient Samples Control Samples - Avg: 0.31 - SD: 0.19 NFL Samples - Avg: 2.8 - SD: 2.9 AD Samples - Avg: 3.1 - SD: 1.8 Previously Unreleased Data
| 39 |

• Next Steps – Continued AD and CTE Validations – Follow - on TauSome study in former NFL players to kick - off in Q2
| 40 |

THERAPEUTIC TECHNOLOGIES TO ADDRESS UNMET NEEDS IN GLOBAL HEALTH & BIODEFENSE
| 41 |

The A e thlon Hemopurifier® A First - In - Class Broad - Spectrum Technology Designed For The Single - Use Removal of Viruses From Blood
| 42 |

The A e thlon Hemopurifier® A Broad - Spectrum Strategy To Address x Drug Resistant Viruses x Emerging Pandemic Threats x Bioterror Threats x Genetically Engineered Viruses
| 43 |

SAVE LIVES
| 44 |

Acknowledgement and Thanks • Team Aethlon • Battelle Memorial Research Institute • Boston University CTE Center • DaVita Med Center Dialysis • Defense Advanced Research Projects Agency (DARPA ) • Frankfurt University Hospital • Medanta Medicity, Fortis and Apollo Hospitals • National Institute of Virology (NIV) Pune, India • Philipps University Marburg • U.S. Army Medical Research Institute for Infectious Diseases (USAMRIID ) • U.S. Centers for Disease Control (CDC)
| 45 |

CONTACT: A e thlon Medical, Inc 9635 Granite Ridge Drive Suite 100 San Diego, California 92123 Jim Joyce Chairman & CEO jj@aethlonmedical.com 858.459.7800 x301
| 46 |