AWARD CONTRACT AND MODIFICATIONS
Published on November 10, 2016
Exhibit 10.1
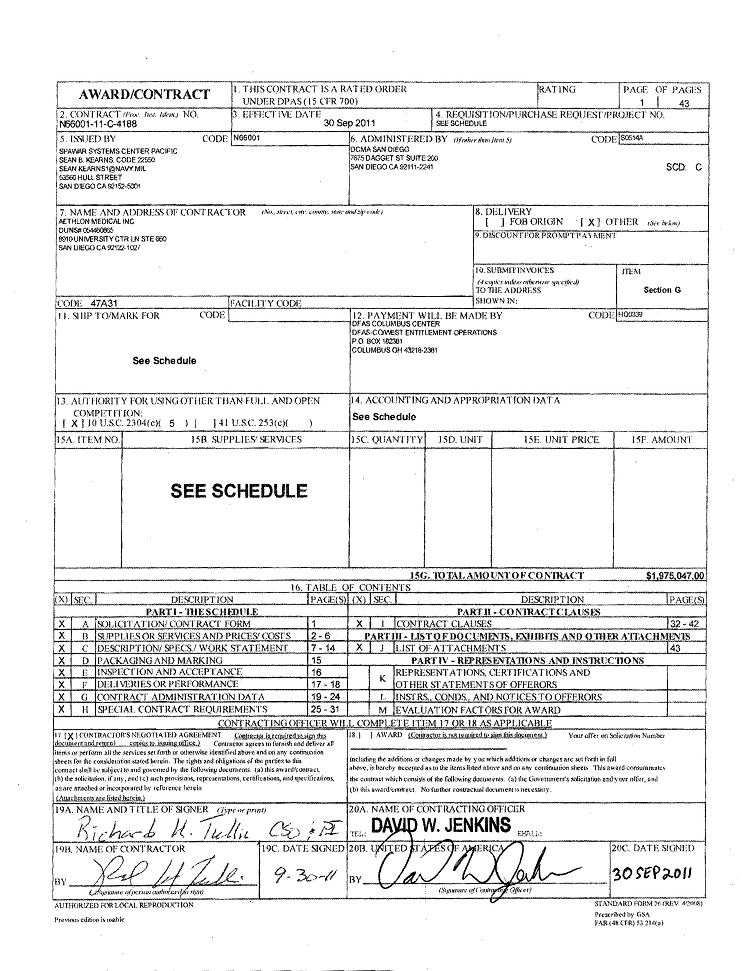
N66001-11-C-4188
Page 2 of 40
Section B - Supplies or Services and Prices
| ITEM NO | SUPPLIES/SERVICES | QUANTITY | UNIT | UNIT PRICE | AMOUNT |
| 0001 | 1 | Lot | $1,975,047.00 | $1,975,047.00 | |
|
RESEARCH FFP Research IAW the SOW (Contained in section C). FOB: Destination
|
|||||
| NET AMT | $1,975,047.00 | ||||
|
PURCHASE REQUEST NUMBER: 1300211787
|
|||||
| 000101 | Funding Information | ACRN | $938,583.00 | ||
| AA | |||||
| ITEM NO | SUPPLIES/SERVICES | QUANTITY | UNIT | UNIT PRICE | AMOUNT |
| 0002 | NSP | ||||
| CDRLs |
N66001-11-C-4188
Page 3 of 40
| ITEM NO | SUPPLIES/SERVICES | QUANTITY | UNIT | UNIT PRICE | AMOUNT |
| 0003 | 1 | Lot | $835,124.00 | $835,124.00 | |
| OPTION | RESEARCH | ||||
| FFP | |||||
| Research IAW the SOW (Contained in section C). | |||||
| FOB: Destination | |||||
| NET AMT | $835,124.00 | ||||
| ITEM NO | SUPPLIES/SERVICES | QUANTITY | UNIT | UNIT PRICE | AMOUNT |
| 0004 | NSP | ||||
| OPTION | CDRLs |
| ITEM NO | SUPPLIES/SERVICES | QUANTITY | UNIT | UNIT PRICE | AMOUNT |
| 0005 | |||||
| OPTION | 1 | Lot | $782,322.00 | $782,322.00 | |
| Human and Animal Use FFP Tasking in SOW section 2.3.2 will not be funded until the contractor obtains all necessary IRB documentation and obtain both institutional and Government (SSC- Pacific) approval in accordance with IRB documentation submission guidance prior to conducting human or animal testing. FOB: Destination |
|||||
| NET AMT | $782,322.00 | ||||
N66001-11-C-4188
Page 4 of 40
| ITEM NO | SUPPLIES/SERVICES | QUANTITY | UNIT | UNIT PRICE | AMOUNT |
| 0006 | 1 | Lot | $1,534,009.00 | $1,534,099.00 | |
| OPTION | RESEARCH | ||||
| FFP | |||||
| Research IAW the SOW (Contained in section C). | |||||
| FOB: Destination | |||||
| NET AMT | $1,534,099.00 | ||||
| ITEM NO | SUPPLIES/SERVICES | QUANTITY | UNIT | UNIT PRICE | AMOUNT |
| 0007 | Lot | NSP | |||
| OPTION | CDRLs |
| ITEM NO | SUPPLIES/SERVICES | QUANTITY | UNIT | UNIT PRICE | AMOUNT |
| 0008 | 1 | Lot | |||
| OPTION | $892,922.00 | $892,922.00 | |||
| Research FFP Research IAW the SOW (Contained in section C). FOB: Destination |
|||||
| NET AMT | $892,922.000 | ||||
N66001-11-C-4188
Page 5 of 40
| ITEM NO | SUPPLIES/SERVICES | QUANTITY | UNIT | UNIT PRICE | AMOUNT |
| 0009 | NSP | ||||
| OPTION | CDRLs |
| ITEM NO | SUPPLIES/SERVICES | QUANTITY | UNIT | UNIT PRICE | AMOUNT |
| 0010 | 1 | Lot | |||
| OPTION | NSP | ||||
| Hardware Deliverable FFP 50 Prototype Optimized Cartridges FOB: Destination |
|||||
| NET AMT | |||||
| ITEM NO | SUPPLIES/SERVICES | QUANTITY | UNIT | UNIT PRICE | AMOUNT |
| 0011 | 1 | Lot | |||
| OPTION | RESEARCH | $774,875.00 | $774,875.00 | ||
| FFP Research IAW the SOW (Contained in section C). FOB: Destination |
|||||
| NET AMT | $774,875.000 | ||||
N66001-11-C-4188
Page 6 of 40
| ITEM NO | SUPPLIES/SERVICES | QUANTITY | UNIT | UNIT PRICE | AMOUNT |
| 0012 | NSP | ||||
| OPTION | CDRLs |
N66001-11-C-4188
Page 7 of 40
Section C - Descriptions and Specifications
STATEMENT OF WORK (SOW)
Statement of Work
Aethlon Medical, Inc.
DATE: 23 September 2011
TITLE: Broad Spectrum Countermeasures for Viral and Bacterial Sepsis using Dialysis-Like Devices
1. Scope
The scope of this effort is to use Aethlon’s ADAPT System as the core technology within an extracorporeal blood purification device that would simultaneously remove: viruses, virally-derived immunosuppressive glycoproteins, and multiple classes of exosomes; complement activation, activation of virus growth (e.g. cytomegalovirus) and TLR activation, all of which have implications to the promotion of the well-being and recovery of wounded warfighters and the prevention of sepsis.
1.1 Introduction
This effort will use the adaptable dialysis-like platform (ADAPT) technology that allows for the selective removal of harmful agents from the entire circulatory system. This revolutionary advance overcomes the limitation of devices that indiscriminately adsorb or solely capture particles by molecule size. The platform will provide an expansive therapeutic filtration mechanism to immobilize multiple affinity agents directed toward precursors to sepsis, bacterial toxins, viral pathogens, and disease enhancing particles transported by exosomes. To insure benefit to wounded warfighters, this effort will advance an innovative strategy that will allow therapy administration without systemic anticoagulation.
The ADAPT platform has been previously used to create a broad-spectrum antiviral device that immobilized one lectin affinity agent, resulting in the effective capture of all tested Category A pathogens, as well as exosomes underlying tuberculosis and cancer. In human studies, this same device, known as the Hemopurifier, consistently provided greater than 50% average viral load reductions during four-hour treatment periods in both hepatitis-C and HIV infected individuals without antiviral drug therapy.
The resulting device would save thousands of military and civilian lives each year. Each of these technology advancements will be integrated into a single cartridge that will provide decision-free and life-saving medical care for the wounded warfighter.
1.2 Background
The goal of the DLT program is to develop a portable device that removes “dirty” blood from the body, separates harmful agents, and returns “clean” blood to the body in a manner similar to dialysis treatment of kidney failure. While the device could have an impact across multiple areas of medicine, the target application for this device is sepsis. The envisioned device can also provide early identification of the presence of a pathogen. Once the presence of pathogens has been confirmed, the DLT device will provide continuous "label-free" removal of pathogens, toxins and activated patient cells without pathogen identification or use of pathogen-specific binding chemistries. As a final step in the treatment process, the DLT device will enable closed-loop therapy based on continuous, reduced dimensionality modeling of patient health. Predictive modeling in this fashion will allow us to identify sepsis early, learn what we need to remove, and direct the most effective intervention to improve patient health. This cycle of sensing, adjustment, estimation, computation, and manipulation will modulate key health parameters faster than the underlying disease process and drive the patient towards a stable, healthy state.
N66001-11-C-4188
Page 8 of 40
2. Technical Requirements
2.1 Human and Animal use
Human use is anticipated in this effort, specifically related to the use of human blood. The contractor shall obtain all necessary Institutional Review Board (IRB) approvals, show proper assurance documentation, and obtain proper approval from the Government officials prior to human use testing. Funds associated with human subjected testing shall not be released until IRB documentation has been provided to SSC’s HRPO and approval to release funds has been obtained.
Animal use is anticipated in this effort. The contractor shall obtain all necessary Institutional Animal Care and Utilization Committee (IACUC) approval and demonstrate this approval to the Government (both ACURO and SSC-Pacific) prior to beginning experimentation with animals. If animal use is no longer anticipated, or changes significantly from the approved IACUC then the Principal Investigator (PI) must submit a letter stating the discontinuation of animal use for this effort and/or receive appropriate authorization for IACUC changes of previously specified protocols. Unless prior approval by DARPA is given IACUC documentation must be provided prior to contract award.
2.2 Base Effort (Year 1)
2.2.1 Subtask 1a: Anticoagulant-free Hemopurification Device
| 2.2.1.1 | Write requirements definition for the extracorporeal blood purification system and acquire necessary equipment. |
| 2.2.1.2 | Fabricate breadboard prototypes for anticoagulation-free anti-sepsis extracorporeal system (ASEPSYS) device. Fabricate prototype blood tubing sets. Acquire anti-thrombogenic surface-modified hollow fiber plasma separators. |
| 2.2.1.3 | Assemble and test breadboard ASEPSYS devices ex vivo with bovine blood. The
test will most likely be conducted using a porcine model where the elapsed time to reach a pre-defined degree of clotting in the
blood treatment device will be compared between the new device and two control groups; one using standard anticoagulant therapy
and one using none. Determine contribution of the following techniques and approaches to eliminating anticoagulants: (1) Backflushing at regular intervals, (2) Turbo loop, (3) Continuous pre-dilution loop using re-captured hydration fluid, (4) Linear vs pulsatile blood flow, (5) Elimination of air/blood interfaces in extracorporeal circuit, (6) Anti-thrombogenic derivatized plasma separation membrane, and (7) Ultra-short half-life anticoagulant nafamostat mesilate. |
| 2.2.1.4 | IRB Documentation Generation: The contractor shall obtain all necessary IRB documentation and obtain both institutional and Government (SSC-Pacific) approval in accordance with IRB documentation submission guidance prior to conducting human subject testing. |
Milestones
| M1: | Demonstrate the effectiveness of the prototype device in preventing platelet activation or clotting in at least a 2 hour blood pumping experiment at 100 mL/hr blood flow. |
2.2.2 Subtask 2: Removal of Sepsis Precursors
| 2.2.2.1 | Begin to develop a device based on Aethlon’s ADAPT system to efficiently capture sepsis precursors identified as potentially important in killing patients undergoing sepsis. The strategy is takes advantage of the flexibility and rapidity of modification of our ADAPT platform system to test any sepsis precursor candidates that circulate in the blood. The sepsis precursors that will be targeted are shown in Table I, in order of importance. No test for the removal of bacterial toxins. No testing for removal of cytokines, since the evidence to date does not support a role for them in death due to sepsis. Additional factors may become known during the grant period and those will also be tested as time and budget permit. |
N66001-11-C-4188
Page 9 of 40
| 2.2.2.2 | Screening Capture Agents: Perform initial screening of the different proposed capture agents by measuring binding affinity and kinetics using surface plasmon resonance (SPR) or biolayer surface interferometry (BLI). |
| 2.2.2.3 | Perform quantitative real time PCR will also be used to measure viral load, and specific DNA or RNA targets. |
Milestones
| M2: | Target capture > 50% in 24 hours for at least 1 target in blood or blood components |
Table I Potential Target Sepsis Precursors and Broad Spectrum Binding Agents
| Group | Factor | Proposed Binding Agents |
| Sepsis related Exosomes | ||
| 1. iNOS exosomes [1-3] | Inducible NO synthase containing exosomes implicated in sepsis | GNA lectin or iNOS Specific antibody |
| 2. Platelet derived exosomes [4, 5] | Exosomes isolated from platelets associated with sepsis | GNA lectin or antibodies |
| 3. Macrophage derived exosomes [6] | Exosomes from cultured macrophages | GNA lectin |
| Other Potential Sepsis Factors | ||
| 4. Complement Activation [7-9] | Humanized Cobra venom factor (CVF) from Incode, CVF is not a toxin | CVF is a stable analog of human complement that neutralizes C3a in animals |
| 5. Bacterial DNA [13-15] | CpG rich DNA activates macrophages via Toll Like Receptors (TLR) | Antisense nuclease resistant DNA analogs, TLRs or specific antibodies |
| 6. Common Sepsis associated Viruses including CMV | Virus blooms in trauma and burn patients [10] | GNA Lectin |
| 7. Protease leakage through ischemic gut (e.g. trypsin) [11, 12] |
Ischemia in the gut leads to protease leakage into the blood and the symptoms of sepsis (reperfusion injury) | Lima bean trypsin inhibitor, Soybean trypsin inhibitor |
N66001-11-C-4188
Page 10 of 40
2.3 Option 1 (Year 2)
2.3.2 Subtask 1a: Anticoagulant-free Hemopurification Device
| 2.3.2.1 | Demonstrate the effectiveness of the prototype device in vivo in animals preventing platelet activation or clotting in at least a 2 hour blood pumping experiment at 75 mL/min blood flow. |
| 2.3.2.2 | Formulate initial design based on work from previous phase. Begin to build and test selected instrument design and tubing sets. |
| 2.3.2.3 | Write and test software. Conduct ergonomic research. Begin discussions with System Integrator. |
Milestone
| M3: | Demonstrate the effectiveness of the prototype device in preventing platelet activation or clotting in at least a 8 hour blood pumping experiment at 500 mL/hr blood flow. |
2.3.3 Subtask 2. Removal of Sepsis Precursors
| 2.3.3.1 | Build the ADAPT capture cartridges with the identified affinity agents. Measure the rate of capture of the specific targets from in ex vivo recirculation experiments from cell culture and blood. |
| 2.3.3.2 | Cartridge construction with optimized affinity matrix design for each potential target. Complete all capture agents screening. Initiate ex vivo capture studies from blood using the optimized cartridges. |
Milestones
| M4: | Target capture > 50% in 24 hours for at least 5 targets in blood or blood components. |
| M5: | Milestone 5: Target capture > 90% in 24 hours for at least 3 targets in blood or blood components. |
NOTE: TASK 2.3.2 SHALL NOT BE EXERCISED AND TASKING FUNDS RELEASED UNTIL IRB DOCUMENTATION AND PROPER IRB APPROVAL HAS BEEN OBTAINED.
2.4 Option 2 (Year 3)
2.4.1 Subtask 1a: Anticoagulant-free Hemopurification Device
| 2.4.1.1 | Collaborate with System Integrator to build final prototypes for in vivo pig testing. |
| 2.4.1.2 | Perform animal tests to confirm the performance of the device in vivo. |
| 2.4.1.3 | Document all adverse events and long term effects of treatment. |
Milestones
| M6: | Demonstrate the effectiveness of the prototype device in preventing platelet activation or clotting in at least a 24 hour blood pumping experiment at 1250 mL/hr blood flow in vivo in pigs. |
2.4.2 Subtask 4: Target Capture in Combined Agent Cartridge
| 2.4.2.1 | Candidate cartridges that demonstrate >90% capture in 24 hours efficacy in binding to individual sepsis precursor targets will move to the next stage. These capture agents will be combined into a single cartridge and retested ex vivo in pig blood or blood components. |
| 2.4.2.2 | Optimize cartridge design in regard to fiber length, diameter and the use of prototype ASEPSYS system. Demonstrate increased capture rates 2-7 fold from the current system in blood or blood components. 2.4.2.3 Perform basic biocompatibility studies to confirm that the combination cartridge does not present any new patient risks that need to be addressed. |
Milestones
| M7: | Target capture > 50% in 24 hours for at least 5 of the 7 targets ex vivo in blood or blood components using the combination cartridge. |
N66001-11-C-4188
Page 11 of 40
| M8: | Optimize cartridge composition for target capture in a single cartridge demonstrating increased capture rates 2-7 fold from the current system in blood or blood components. |
| M9: | Target capture > 90% in 24 hours (12 months) for at least 5 of the 7 targets ex vivo in blood or blood components using the optimized cartridge |
| M10: | Pass biocompatibility tests for the combination ADAPT device. |
2.5 Option 3 (Year 4)
2.5.1 Subtask 1a: Anticoagulant-free Hemopurification Device
| 2.5.1.1 | System integrator implements design modifications emanating from pig experiments. |
| 2.5.1.2 | Collaborate with System Integrator in conducting verification and validation testing and collecting all remaining data required for IDE submission (e.g. biocompatibility, electromagnetic interference, electromagnetic susceptibility, software V&V, etc.). |
| 2.5.1.3 | Make additional cartridge or device modification as required by system integrator. |
Milestones
M11: Demonstrate the effectiveness of the newest device design in preventing blood clotting in a 24 hour blood pumping experiment at 1275 mL/hr blood flow in vivo.
2.5.2 Subtask 4: Target Capture in Combined Agent Cartridge
| 2.5.2.1 | Determine the in vivo efficiency of an optimized combined clearance cartridge incorporating all the successful capture agents. |
| 2.5.2.2 | Finish construction and delivery of 50 prototype cartridges for testing by the system integrator. The |
| cartridges | will need to be made available (packaged, labeled, sterilized and qualified) to the system integrator. |
| 2.5.2.3 | Perform basic biocompabability tests for the combination ADAPT device to confirm the combination cartridge does not present any new patient risk. |
Milestones
| M12: | Complete studies in septic pig models with optimized combination cartridge for >90% clearance of at least 4 of the 7 sepsis marker targets in 24 hours (12 months) |
| M13: | Construct and deliver of 50 prototype cartridges for testing by the system integrator. |
2.6 Option 4 (Year 5)
2.6.1 Subtask 5: Testing of final product by System Integrator
| 2.6.1.1 | System Integrator approval of ASEPSYS device for portable blood pump without the need for systemic anticoagulation. |
| 2.6.1.2 | System Integrator testing of the ADAPT treatment cartridge for reducing sepsis related death by >20% in a septic animal pig model. |
| 2.6.1.3 | Prepare and submit IDE proposal for sepsis treatment based on previously approved IDE. |
| 2.6.1.4 | Prepare and present Final report for DARPA. |
Milestones
| M13: | System Integrator approval of a sepsis precursor ADAPT treatment cartridge for reducing sepsis related death by >20% in a septic animal pig model. |
| M14: | System integrator acceptance of the ASEPSYS anticoagulation device as the blood pump that can avoid the need for systemic anticoagulation. |
N66001-11-C-4188
Page 12 of 40
3.0 Program Management and Reviews
3.1 Program Management Plan
The contractor shall develop a Program Management Plan. A graphical representation of this plan (Gantt chart is one example) identifying major tasks and their task leaders, milestones of the major task and their completion dates shall be generated. In addition, a graphical representation of budget shall be generated.
3.2 Kick-off Meeting
The contractor shall participate in a kick-off meeting within 60 days of contract award. The purpose of this meeting is to introduce key program personnel, discuss the proposed tasking, present the program schedule and milestones and the initial Program Management Plan.
3.3 Quarterly Reviews
The contractor shall hold quarterly reviews for the duration of this effort. The purpose of these reviews is to present a summary of work completed and milestones met, discuss any problems encountered, update the program schedule, present the program financial status, and discuss remaining work.
3.4 Final Contract Review
A final contract review held in place of the last quarterly review shall be hosted by the principal contractor. The purpose of this review is to present a summary of all work completed and milestones accomplished and to discuss any relevant future efforts similar to the contract that may be pursued.
4.0 Deliverables
The reports and presentation materials are to be delivered in accordance with the contract CDRLs.
CLAUSES INCORPORATED BY FULL TEXT
5252.227-9211 PROCEDURES FOR CONTROLLING TECHNICAL DOCUMENTS UNDER SPAWARSYSCEN PACIFIC CONTRACTS (NOV 2008)
The Contractor shall comply with DOD Directive 5230.25 and the information provided herein when the Government provides the Contractor with technical data.
(a) Location of distribution statement, export warning notice, and destruction notice (classified and unclassified technical documents).
(1) Standard written or printed material with covers and/or title pages: Statement(s) to be printed, typed, or stamped on the front cover and title page.
(2) Technical documents without covers or title pages: Statement(s) to be typed, printed, or stamped on the first page of the document.
(3) Deck of punched or aperture cards: Statement(s) to be typed, printed, or stamped on face of first and last card and on top of deck.
(4) Magnetic tape, cassette, or disk: Statement(s) to be typed, stamped, or printed on a label applied to outside of material. The first page of the resulting hard-copy report or computer printout is also marked with applicable statement(s).
(5) Microfilm: Statement(s) to be typed, stamped, or printed on outside of jacket or canister housing the material. The first page of the resulting hard-copy report or first frame is also marked with applicable statement(s). The headers for microfiche must carry an abbreviated version of the statement(s).
(6) Drawings: Applicable statement(s) to be typed, stamped, or printed near the title block.
(b) Safeguarding of Unclassified, Limited-Access Documents (for classified documents see NOSCINST 5500.1A).
(1) Normal working hours: Limited-access documents and those that have not yet been reviewed cannot be left unattended in work areas accessible to non-DoD employees.
(2) After normal working hours: Limited-access documents and those that have not yet been reviewed should be placed in locked files, desks, or similar containers. If this is not possible, locked offices or buildings are adequate.
N66001-11-C-4188
Page 13 of 40
(3) Additional guidance for safeguarding limited-access media processed by an IT system, activity, or network can be found in OPNAVINST 5239.1A.
(c) Destruction of Unclassified, Limited-Access Documents. Destroy by any method that will prevent disclosure of contents or reconstruction of the material. Examples of such destruction methods follow:
(1) Printed document, deck of punched or aperture cards, computer printout, and drawings: Destroy by tearing each copy into pieces to preclude reconstruction and placing the pieces in regular trash containers or send to the Mail Room Branch for destruction.
(2) Magnetic tape, cassette, or disk: Destroy by erasing the magnetic storage media.
(3) Microfilm: Destroy by cutting into small pieces or send to the mailroom for destruction.
(d) Safeguarding of Classified Documents: See NOSCINST 5500.1A.
(e) Destruction of Classified Documents: See NOSCINST 5500.1A.
(End of specification)
5252.237-9601 KEY PERSONNEL (DEC 1999)
(a) The offeror agrees to assign to this contract those key personnel listed in paragraph (d) below. No substitutions shall be made except in accordance with this clause.
(b) The offeror agrees that during the first 6 months of the contract performance period no personnel substitutions will be permitted unless such substitutions are necessitated by an individual's sudden illness, death or termination of employment. In any of these events, the contractor shall promptly notify the Contracting Officer and provide the information required by paragraph (c) below. After the initial 6 month period, all proposed substitutions must be submitted in writing, at least fifteen (15) days (thirty (30) days if a security clearance is to be obtained) in advance of the proposed substitutions to the contracting officer. These substitution requests shall provide the information required by paragraph (c) below.
(c) All requests for approval of substitutions under this contract must be in writing and provide a detailed explanation of the circumstances necessitating the proposed substitutions. They must contain a complete resume for the proposed substitute or addition, and any other information requested by the Contracting Officer or needed by him to approve or disapprove the proposed substitutions. All substitutions proposed during the duration of this contract must have qualifications of the person being replaced. The Contracting Officer or his authorized representative will evaluate such requests and promptly notify the contractor of his approval or disapproval thereof in writing.
(d) List of Key Personnel
| NAME | CONTRACT LABOR CATEGORY |
| Richard H. Tullis, PhD | Chief Science Officer |
(e) If the Contracting Officer determines that suitable and timely replacement of key personnel who have been reassigned, terminated or have otherwise become unavailable for the contract work is not reasonably forthcoming or that the resultant reduction of productive effort would be so substantial as to impair the successful completion of the contract or the service order, the contract may be terminated by the Contracting Officer for default or for the convenience of the Government, as appropriate. In addition, if the Contractor is found at fault for the condition, the Contracting Officer may elect to equitably decrease the contract price or fixed fee to compensate the Government for any resultant delay, loss or damage.
N66001-11-C-4188
Page 14 of 40
(f) If the offeror wishes to add personnel to be used in a labor category he shall employ the procedures outlined in paragraph (c) above. Adding personnel will only be permitted in the event of an indefinite quantity contract, where the Government has issued a delivery order for labor hours that would exceed a normal forty hour week if performed only by the number of employees originally proposed.
(End of clause)
N66001-11-C-4188
Page 15 of 40
Section D - Packaging and Marking
CLAUSES INCORPORATED BY FULL TEXT
252.235-7010 ACKNOWLEDGMENT OF SUPPORT AND DISCLAIMER (MAY 1995)
(a) The Contractor shall include an acknowledgment of the Government’s support in the publication of any material based on or developed under this contract, stated in the following terms: This material is based upon work supported by the (name of contracting agency(ies)) under Contract No. (Contracting agency(ies) contract numbers(s).
(b) All material, except scientific articles or papers published in scientific journals, must, in addition to any notices or disclaimers by the Contractor, also contain the following disclaimer: Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the (name of contracting agency(ies).
(End of clause)
N66001-11-C-4188
Page 16 of 40
Section E - Inspection and Acceptance
INSPECTION AND ACCEPTANCE TERMS
Supplies/services will be inspected/accepted at:
| CLIN | INSPECT AT | INSPECT BY | ACCEPT AT | ACCEPT BY |
| 0001 | Destination | Government | Destination | Government |
| 0002 | Destination | Government | Destination | Government |
| 0003 | Destination | Government | Destination | Government |
| 0004 | Destination | Government | Destination | Government |
| 0005 | Destination | Government | Destination | Government |
| 0006 | Destination | Government | Destination | Government |
| 0007 | Destination | Government | Destination | Government |
| 0008 | Destination | Government | Destination | Government |
| 0009 | Destination | Government | Destination | Government |
| 0010 | Destination | Government | Destination | Government |
| 0011 | Destination | Government | Destination | Government |
| 0012 | Destination | Government | Destination | Government |
CLAUSES INCORPORATED BY REFERENCE
| 52.246-7 | Inspection Of Research And Development Fixed Price | AUG 1996 |
| 252.246-7000 | Material Inspection And Receiving Report | MAR 2008 |
N66001-11-C-4188
Page 17 of 40
Section F - Deliveries or Performance
DELIVERY INFORMATION
| CLIN | PERIOD OF | SHIP TO ADDRESS | UIC | |
| PERFORMANCE | QUANTITY | |||
| 0001 | 12 MONTHS AFTER | SPAWAR SYSTEMS CENTER | N66001 | |
| DATE OF CONTRACT | RECEIVING OFFICER | |||
| AWARD | N/A | 4297 PACIFIC HIGHWAY, BLDG 7 SAN DIEGO CA 92110-5000 | ||
| 619-553-1251 | ||||
| FOB: Destination | ||||
| 0002 | 12 MONTHS AFTER | (SAME AS PREVIOUS LOCATION) | N66001 | |
| DATE OF CONTRACT | N/A | FOB: Destination | ||
| AWARD | ||||
| 0003 | 12 MONTHS AFTER | (SAME AS PREVIOUS LOCATION) | N66001 | |
| DATE OF OPTION I | N/A | FOB: Destination | ||
| AWARD | ||||
| 0004 | 12 MONTHS AFTER | (SAME AS PREVIOUS LOCATION) | N66001 | |
| DATE OF OPTION I | N/A | FOB: Destination | ||
| AWARD | ||||
| 0005 | 12 MONTHS AFTER | (SAME AS PREVIOUS LOCATION) | N66001 | |
| DATE OF OPTION I | 1 | FOB: Destination | ||
| AWARD | ||||
| 0006 | 12 MONTHS AFTER | (SAME AS PREVIOUS LOCATION) | N66001 | |
| DATE OF OPTION II | N/A | FOB: Destination | ||
| AWARD | ||||
| 0007 | 12 MONTHS AFTER | (SAME AS PREVIOUS LOCATION) | N66001 | |
| DATE OF OPTION II | N/A | FOB: Destination | ||
| AWARD | ||||
| 0008 | 12 MONTHS AFTER | (SAME AS PREVIOUS LOCATION) | N66001 | |
| DATE OF OPTION III | N/A | FOB: Destination | ||
| AWARD | ||||
| 0009 | 12 MONTHS AFTER | (SAME AS PREVIOUS LOCATION) | N66001 | |
| DATE OF OPTION III | N/A | FOB: Destination | ||
| AWARD | ||||
| 0010 | 12 MONTHS AFTER | (SAME AS PREVIOUS LOCATION) | N66001 | |
| DATE OF OPTION III | 1 | FOB: Destination | ||
| AWARD |
N66001-11-C-4188
Page 18 of 40
| 0011 | 12 MONTHS AFTER | (SAME AS PREVIOUS LOCATION) | N66001 | |
| DATE OF OPTION IV | N/A | FOB: Destination | ||
| AWARD | ||||
| 0012 | 12 MONTHS AFTER | (SAME AS PREVIOUS LOCATION) | N66001 | |
| DATE OF OPTION IV | N/A | FOB: Destination | ||
| AWARD |
CLAUSES INCORPORATED BY REFERENCE
| 52.242-15 | Stop-Work Order | AUG 1989 |
| 52.242-15 Alt I | Stop-Work Order (Aug 1989) - Alternate I | APR 1984 |
| 52.247-34 | F.O.B. Destination | NOV 1991 |
N66001-11-C-4188
Page 19 of 40
Section G - Contract Administration Data
ACCOUNTING AND APPROPRIATION DATA
AA: 9710400 1320 595 0P1D1 0 2525DP AM 179166 1101E S12136
AMOUNT: $938,583.00
CIN 130021178700002: $938,583.00
CLAUSES INCORPORATED BY FULL TEXT
252.204-0007 CONTRACT-WIDE: SEQUENTIAL ACRN ORDER. (SEP 2009)
The payment office shall make payment in sequential ACRN order within the contract or order, exhausting all funds in the previous ACRN before paying from the next ACRN using the following sequential order: alpha/alpha; alpha/numeric; numeric/alpha; and numeric/numeric.
(End of clause)
5252.201-9201 DESIGNATION OF CONTRACTING OFFICER'S REPRESENTATIVE (MAR 2006)
(a) The Contracting Officer hereby appoints the following individual as Contracting Officer’s Representative(s) (COR) for this contract/order:
CONTRACTING OFFICER REPRESENTATIVE
Name: John Rockway
Code: 52260
Address: 53560 Hull Street, San Diego, CA 92152-5001
Phone Number: 619-204-0988
E-mail: john.rockway@navy.mil
(b) It is emphasized that only the Contracting Officer has the authority to modify the terms of the contract, therefore, in no event will any understanding agreement, modification, change order, or other matter deviating from the terms of the basic contract between the Contractor and any other person be effective or binding on the Government. When/If, in the opinion of the Contractor, an effort outside the existing scope of the contract is requested, the Contractor shall promptly notify the PCO in writing. No action shall be taken by the Contractor unless the Procuring Contracting Officer (PCO) or the Administrative Contracting Officer (ACO) has issued a contractual change.
N66001-11-C-4188
Page 20 of 40
5252.216-9210 TYPE OF CONTRACT (DEC 1999)
This is a Firm-Fixed Price (FFP) Completion contract.
(End of clause)
5252.227-9213 PATENT MATTERS POINT OF CONTACT (OCT 2008)
The Point of Contact regarding Patent Matters for this contract is:
OFFICE OF PATENT COUNSEL / CODE 360012
SPAWARSYSCEN
53560 HULL STREET
SAN DIEGO, CA 92152-5001
(619) 553-3001
Do not submit interim and final invention reports to this address. See the clause at 5252.227-9206 for the proper address.
(End of clause)
5252.232-9208 INVOICING INSTRUCTIONS FOR SERVICES USING WIDE AREA WORK FLOW (WAWF) (APR 2009)
(a) Invoices for services rendered under this contract shall be submitted electronically through the Wide Area Work Flow-Receipt and Acceptance (WAWF). The contractor shall submit invoices for payment per contract terms. The Government shall process invoices for payment per contract terms.
(b) The vendor shall have their Cage Code activated by calling 1-866-618-5988 and selecting option 2. Once activated, the vendor shall self-register at the WAWF website at https://wawf.eb.mil. Vendor training is available on the internet at https://wawftraining.eb.mil. WAWF Vendor “Quick Reference” Guides are located at the following web site: http://acquisition.navy.mil/rda/home/acquisitiononesource/ebusiness/donebusinesssolutions/wa wfoverview/vendorinformation
(c) Cost back-up documentation (such as delivery receipts, labor hours & material/travel costs etc.) shall be included and attached to the invoice in WAWF. Attachments created with any Microsoft Office product or Adobe (.pdf files) are attachable to the invoice in WAWF. The total size limit for files per invoice is 5 megabytes. A separate copy shall be sent to the COR/TOM.
(d) Contractors approved by DCAA for direct billing will not process vouchers through DCAA, but may submit directly to DFAS. Vendors MUST still provide a copy of the invoice and any applicable cost back-up documentation supporting payment to the Acceptor/Contracting Officer's Representative (COR) if applicable. Additionally, a copy of the invoice(s) and attachment(s) at time of submission in WAWF shall also be provided to each point of contact identified in section (g) of this clause by email. If the invoice and/or receiving report are delivered in the email as an attachment it must be provided as a .PDF, Microsoft Office product or other mutually agreed upon form between the Contracting Officer and vendor.
N66001-11-C-4188
Page 21 of 40
(e) A separate invoice will be prepared no more frequently than for every two weeks. Do not combine the payment claims for services provided under this contract.
(f) The following information is provided for completion and routing of the invoice in WAWF:
| WAWF Invoice Type | 2-n-1 (Services Only) |
| Issuing Office DODAAC | See Block 5 of the SF26 |
| Admin DODAAC | See Block 6 of the SF26 |
| Inspector DODAAC (if applicable) | N66001 |
| Inspector Contact Information | See Clause 5252.201-9201 |
| Service Acceptor DODAAC | N66001 |
| Acceptor Contact Information | See Clause 5252.201-9201 |
| COR Contact Information | See Clause 5252.201-9201 |
| DCAA Auditor DoDAAC : | N/A |
| Service Approver DoDAAC : | See Block 6 of the SF26 |
| PAY DODAAC | See Block 12 of the SF26 |
(g) After submitting the document(s) to WAWF, click on “Send More Email Notifications” and add the acceptor/receiver email addresses noted below in the email address blocks. The contractor shall, at a minimum, include the COR, Receiver, and Acceptor. This additional notification to the government is necessary to ensure that the acceptor/receiver is aware that the invoice documents have been submitted into WAWF:
| Send Additional Email Notification(s) to: | |||
| Name | Phone | Role | |
| See Clause 5252.201-9201 | COR | ||
(End of clause)
5252.243-9600 AUTHORIZED CHANGES ONLY BY THE CONTRACTING OFFICER (JAN 1992)
(a) Except as specified in paragraph (b) below, no order, statement, or conduct of Government personnel who visit the Contractor’s facilities or in any other manner communicates with Contractor personnel during the performance of this contract shall constitute a change under the Changes clause of this contract.
(b) The Contractor shall not comply with any order, direction or request of Government personnel unless it is issued in writing and signed by the Contracting Officer, or is pursuant to specific authority otherwise included as a part of this contract.
(c) The Contracting Officer is the only person authorized to approve changes in any of the requirements of this contract and notwithstanding provisions contained elsewhere in this contract, the said authority remains solely the Contracting Officer’s. In the event the contractor effects any change at the direction of any person other than the Contracting Officer, the change will be considered to have been made without authority and no adjustment will be made in the contract price to cover any increase in charges incurred as a result thereof.
(End of clause)
N66001-11-C-4188
Page 22 of 40
ADMINISTRATIVE INSTRUCTIONS
INCORPORATION OF REPRESENTATIONS AND CERTIFICATIONS
All representations and certifications and other written statements made by the contractor in response to Section K of the solicitation or at the request of the contracting officer which are incident to the award of the contract or modification of this contract, are hereby incorporated by reference with the same force and effect as if they were given in full text.
(End of Instruction)
MARKING OF SHIPMENT
Each shipment of material and/or data shall be clearly marked to show the following information:
| SHIP TO: | MARK FOR: |
| RECEIVING OFFICER | Contract #: N66001-11-C-4188 |
| Item #: ALL | |
| Receiving Officer Code: 56506 |
The receiving office is located at 4297 Pacific Highway, Bldg. 7, San Diego, CA 92110-5000 and is open for deliveries Monday through Thursday from 6:30 AM until 4:00 PM and Fridays 6:30 AM to 3:00 PM.
(End of Instruction)
AGREEMENT TO LICENSE--NO IMPLIED LICENSE
(a) Except as provided in paragraph (b) below:
(1) Aethlon Medical, Inc. shall obtain a license from the U.S. Government under the following U.S. patents, patent applications and all patents issuing thereon, and under all patents that may issue and patent applications that may be filed on the following invention disclosures, on reasonable terms and conditions, consistent with law, regulation, and Navy policy prior to any manufacture, use, sale, lease, license, or conveyance of any kind of any process, machine, manufacture, or composition of matter that would, absent such license, infringe any claim of such patent(s)/application(s):
NONE KNOWN AT THIS TIME
(2) Nothing in this contract shall release Aethlon Medical, Inc. from any obligation of or duty under any other Government contract; nor shall it grant to or confer upon Aethlon Medical, Inc. any rights, express or implied,
(i) to any invention other than a Subject Invention,
(ii) under any patent application or patent assigned to the U.S. Government that is dominant over a patent protecting a Subject Invention,
(iii) under any patent application or patent assigned to the U.S. Government protecting an invention other than a Subject Invention, or
(iv) under the U.S. patent(s)/patent application(s) identified in paragraph (a)(1) above.
(b) No license from the U.S. Government shall be required for research, development, test and evaluation to be performed by Aethlon Medical, Inc. under this contract.
(End of Instruction)
APPLICATION OF DFARS 252.227-7013 AND 252.227-7015 TECHNICAL DATA CLAUSES
The DFARS 252.227-7015, Technical Data--Commercial Items, clause applies to technical data that pertains to a “commercial item” as defined in the DFARS 252.227-7015 clause. The DFARS 252.227-7013, Rights in Technical Data--Noncommercial Items, clause applies to all other technical data.
(End of Instruction)
N66001-11-C-4188
Page 23 of 40
DISSEMINATION NOTICES FOR TECHNICAL DOCUMENTS PREPARED UNDER SPAWARSYSCEN
PACIFIC CONTRACTS (NOV 2008)
(a) Unless otherwise specified, all classified and unclassified technical documents generated under this contract must carry the following statements:
(1) Do not distribute to DTIC or other data depositories.
(2) Distribution authorized to DOD components only; premature dissemination [Contractor to insert a date which will be determined by the Program Manager and affixed by the Contractor]. Other requests shall be referred to the Space and Naval Warfare Systems Center, Code 2015, San Diego, CA 92152-5001.
(b) The Contractor shall place the above statements on the original and all copies before being delivered to the shipping address in Section F as follows:
(1) Standard Written or Printed material with Covers and/or Title Pages: Statement(s) to be printed, typed, or stamped on front cover and title page.
(2) Technical Documents Without Covers or Title Pages: Statement(s) to be typed, printed, or stamped on first page of the document.
(3) Drawing: Applicable statement(s) to be typed, printed, or stamped near the title block.
(4) Magnetic Tape, Cassette, or Disk: Statement(s) to be typed, printed, or stamped on a label applied to outside of material. The first page of the resulting hard-copy report or computer printout report is also marked with applicable statement(s).
(5) Microfilm: Statement(s) typed, printed, or stamped on outside of jacket or canister housing the material. The first page of resulting hard-copy report or first frame is also marked with applicable statement(s). The headers for microfiche must carry an abbreviated version of the statement(s).
(6) Deck of Punched or Aperture Cards: Statement(s) to be typed, stamped, or printed on face of first and last card and on top of deck.
(End of Instruction)
EXPORT CONTROL (DARPA)
Should this project develop beyond fundamental research (basic and applied research ordinarily published and shared broadly within the scientific community) with military or dual-use applications the following apply:
(1) The Contractor shall comply with all U.S. export control laws and regulations, including the International Traffic in Arms Regulations (ITAR), 22 CFR Parts 120 through 130, and the Export Administration Regulations (EAR), 15 CFR Parts 730 through 799, in the performance of this contract. In the absence of available license exemptions/exceptions the Contractor shall be responsible for obtaining the appropriate licenses or other approvals, if required, for exports of (including deemed exports) hardware, technical data, and software, or for the provision of technical assistance.
(2) The Contractor shall be responsible for obtaining export licenses, if required, before utilizing foreign persons in the performance of this contract, including instances where the work is to be performed on-site at any Government installation (whether in or outside the United States), where the foreign person will have access to export-controlled technologies, including technical data or software.
(3) The Contractor shall be responsible for all regulatory record keeping requirements associated with the use of licenses and license exemptions/exceptions.
(4) The Contractor shall be responsible for ensuring that the provisions of this clause apply to its subcontractors.
(End of instruction)
N66001-11-C-4188
Page 24 of 40
Section H - Special Contract Requirements
CLAUSES INCORPORATED BY FULL TEXT
5252.209-9206 EMPLOYMENT OF NAVY PERSONNEL RESTRICTED (DEC 1999)
In performing this contract, the Contractor will not use as a consultant or employ (on either a full or part-time basis) any active duty Navy personnel (civilian or military) without the prior approval of the Contracting Officer. Such approval may be given only in circumstances where it is clear that no law and no DOD or Navy instructions, regulations, or policies might possibly be contravened and no appearance of a conflict of interest will result.
(End of clause)
5252.227-9205 RIGHTS IN MASK WORKS (DEC 2002)
(a) Definitions.
As defined in 17 U.S.C. §901--
“Semiconductor chip product” is the final or intermediate form of any product--
(A) having two or more layers of metallic, insulating, or semiconductor material, deposited or otherwise placed on, or etched away or otherwise removed from, a piece of semiconductor material in accordance with a predetermined pattern; and
(B) intended to perform electronic circuit functions.
“Mask work” is a series of related images, however fixed or encoded--
(A) having, or representing the predetermined, three-dimensional pattern of metallic, insulating, or semiconductor material present or removed from the layers of a semiconductor chip product; and
(B) in which series the relation of the images to one another is that each image has the pattern of the surface of one form of the semiconductor chip product.
(b) For any and every mask work generated in the performance of work under this contract, the contractor grants to the Government a non-exclusive, irrevocable, royalty free, worldwide license to:
(1) reproduce or have reproduced the mask work by optical, electronic, or any other means; and
(2) import or distribute or have imported or distributed a semiconductor chip product in which the mask work is embodied.
(c) The contractor shall include this clause, suitably modified to replace “contractor” with “subcontractor” in all subcontracts, regardless of tier, in which a mask work is likely to be created in the performance of the work under the subcontract. The contractor shall not obtain rights in the subcontractor’s mask works as any part of the consideration for awarding the subcontract.
(d) This license is specific to mask work rights and shall not be construed to broaden any proprietary rights to technical data or computer software.
(End of clause)
N66001-11-C-4188
Page 25 of 40
5252.227-9206 SUBMISSION OF INTERIM AND FINAL INVENTION REPORTS AND NOTIFICATION OF ALL SUBCONTRACTS FOR EXPERIMENTAL, DEVELOPMENTAL, OR RESEARCH WORK (OCT 2008)
(a) This contract contains either FAR 52.227-11 “Patent Rights--Ownership by the Contractor” clause and DFARS 252.227-7039 “Patents--Reporting of Subject Inventions” or DFARS 252.227-7038 “Patent Rights--Ownership by the Contractor (Large Business)” clause, or FAR 52.227-13 “Patent Rights--Ownership by the Government” clause.
(b) Under these clauses, the Contractor is required to submit interim and final invention reports and notification to the Government of all subcontracts for experimental, developmental, or research work. The interim and final invention reports and notification of all subcontracts for experimental, developmental, or research work may be submitted on DD Form 882 “Report of Inventions and Subcontracts.”
(c) The Contractor shall submit interim and final invention reports and notification of all subcontracts for experimental, developmental, or research work, including negative reports, to:
CONTRACT CLOSEOUT / CODE 23100
SPAWARSYSCEN PACIFIC
53560 HULL STREET
SAN DIEGO, CA 92152-5001
(d) The SPAWARSYSCEN Pacific Office of Patent Counsel, Code 360012, will represent the Contracting Officer with regard to invention reporting matters arising under the contract.
(End of clause)
5252.227-9207 LIMITED RELEASE OF CONTRACTOR CONFIDENTIAL BUSINESS INFORMATION (APRIL 2010)
(a) Definition.
“Confidential Business Information,” (Information) as used in this clause, is defined as all forms and types of financial, business, economic or other types of information other than technical data or computer software/computer software documentation, whether tangible or intangible, and whether or how stored, compiled, or memorialized physically, electronically, graphically, photographically, or in writing if -- (1) the owner thereof has taken reasonable measures to keep such Information secret, and (2) the Information derives independent economic value, actual or potential from not being generally known to, and not being readily ascertainable through proper means by, the public. Information does not include technical data, as that term is defined in DFARS 252.227-7013(a)(14), 252.227-7015(a)(4), and 252.227-7018(a)(19). Similarly, Information does not include computer software/computer software documentation, as those terms are defined in DFARS 252.227-7014(a)(4) and 252.227-7018(a)(4).
(b) The Space and Naval Warfare Systems Command (SPAWAR) may release to individuals employed by SPAWAR support contractors and their subcontractors Information submitted by the contractor or its subcontractors pursuant to the provisions of this contract. Information that would ordinarily be entitled to confidential treatment may be included in the Information released to these individuals. Accordingly, by submission of a proposal or execution of this contract, the offeror or contractor and its subcontractors consent to a limited release of its Information, but only for purposes as described in paragraph (c) of this clause.
(c) Circumstances where SPAWAR may release the contractor’s or subcontractors’ Information include the following:
(1) To other SPAWAR contractors and subcontractors, and their employees tasked with assisting SPAWAR in handling and processing Information and documents in the administration of SPAWAR contracts, such as file room management and contract closeout; and,
N66001-11-C-4188
Page 26 of 40
(2) To SPAWAR contractors and subcontractors, and their employees tasked with assisting SPAWAR in accounting support services, including access to cost-reimbursement vouchers.
(d) SPAWAR recognizes its obligation to protect the contractor and its subcontractors from competitive harm that could result from the release of such Information. SPAWAR will permit the limited release of Information under paragraphs (c)(1) and (c)(2) only under the following conditions:
(1) SPAWAR determines that access is required by other SPAWAR contractors and their subcontractors to perform the tasks described in paragraphs (c)(1) and (c)(2);
(2) Access to Information is restricted to individuals with a bona fide need to possess;
(3) Contractors and their subcontractors having access to Information have agreed under their contract or a separate corporate non-disclosure agreement to provide the same level of protection to the Information that would be provided by SPAWAR employees. Such contract terms or separate corporate non-disclosure agreement shall require the contractors and subcontractors to train their employees on how to properly handle the Information to which they will have access, and to have their employees sign company non disclosure agreements certifying that they understand the sensitive nature of the Information and that unauthorized use of the Information could expose their company to significant liability. Copies of such employee non disclosure agreements shall be provided to the Government;
(4) SPAWAR contractors and their subcontractors performing the tasks described in paragraphs (c)(1) or (c)(2) have agreed under their contract or a separate non-disclosure agreement to not use the Information for any purpose other than performing the tasks described in paragraphs (c)(1) and (c)(2); and,
(5) Before releasing the Information to a non-Government person to perform the tasks described in paragraphs (c)(1) and (c)(2), SPAWAR shall provide the contractor a list of the company names to which access is being granted, along with a Point of Contact for those entities.
(e) SPAWAR’s responsibilities under the Freedom of Information Act are not affected by this clause.
(f) The contractor agrees to include, and require inclusion of, this clause in all subcontracts at any tier that requires the furnishing of Information.
(End of clause)
5252.231-9200 REIMBURSEMENT OF TRAVEL COSTS (JAN
2006)
(a) Contractor Request and Government Approval of Travel
Any travel under this contract must be specifically requested in writing, by the contractor prior to incurring any travel costs. If this contract is a definite or indefinite delivery contract, then the written Government authorization will be by task/delivery orders issued by the Ordering Officer or by a modification to an issued task/delivery order. If this contract is not a definite or indefinite delivery contract, then the written Government authorization will be by written notice of approval from the Contracting Officer’s Representative (COR). The request shall include as a minimum, the following:
(1) Contract number
(2) Date, time, and place of proposed travel
(3) Purpose of travel and how it relates to the contract
(4) Contractor’s estimated cost of travel
(5) Name(s) of individual(s) traveling and;
(6) A breakdown of estimated travel and per diem charges.
N66001-11-C-4188
Page 27 of 40
The contractor shall submit the travel request in writing to the Contracting Officer’s Representative (COR). The COR shall review and approve/disapprove (as appropriate) all travel requests submitted giving written notice of such approval or disapproval to the contractor.
(b) General
(1) The costs for travel, subsistence, and lodging shall be reimbursed to the contractor only to the extent that it is necessary and authorized for performance of the work under this contract. The costs for travel, subsistence, and lodging shall be reimbursed to the contractor in accordance with the Federal Acquisition Regulation (FAR) 31.205-46, which is incorporated by reference into this contract. As specified in FAR 31.205-46(a) (2), reimbursement for the costs incurred for lodging, meals and incidental expenses (as defined in the travel regulations cited subparagraphs (b)(1)(i) through (b)(1)(iii) below) shall be considered to be reasonable and allowable only to the extent that they do not exceed on a daily basis the maximum per diem rates in effect at the time of travel as set forth in the following:
(i) Federal Travel Regulation prescribed by the General Services Administration for travel in the contiguous 48 United States;
(ii) Joint Travel Regulation, Volume 2, DoD Civilian Personnel, Appendix A, prescribed by the Department of Defense for travel in Alaska, Hawaii, The Commonwealth of Puerto Rico, and the territories and possessions of the United States; or
(iii) Standardized Regulations, (Government Civilians, Foreign Areas), Section 925, “Maximum Travel Per Diem Allowances in Foreign Areas” prescribed by the Department of State, for travel in areas not covered in the travel regulations cited in subparagraphs (b)(1)(i) and (b)(1)(ii) above.
(2) Personnel in travel status from and to the contractor’s place of business and designated work site or vice versa, shall be considered to be performing work under the contract, and contractor shall bill such travel time at the straight (regular) time rate; however, such billing shall not exceed eight hours per person for any one person while in travel status during one calendar day.
(c) Per Diem
(1) The contractor shall not be paid per diem for contractor personnel who reside in the metropolitan area in which the tasks are being performed. Per diem shall not be paid on services performed at contractor’s home facility and at any facility required by the contract, or at any location within a radius of 50 miles from the contractor’s home facility and any facility required by this contract.
(2) Costs for subsistence and lodging shall be paid to the contractor only to the extent that overnight stay is necessary and authorized in writing by the Government for performance of the work under this contract per paragraph (a). When authorized, per diem shall be paid by the contractor to its employees at a rate not to exceed the rate specified in the travel regulations cited in FAR 31.205-46(a)(2) and authorized in writing by the Government. The authorized per diem rate shall be the same as the prevailing locality per diem rate.
(3) Reimbursement to the contractor for per diem shall be limited to payments to employees not to exceed the authorized per diem and as authorized in writing by the Government per paragraph (a). Fractional parts of a day shall be payable on a prorated basis for purposes of billing for per diem charges attributed to subsistence on days of travel. The departure day from the Permanent Duty Station (PDS) and return day to the PDS shall be 75% of the applicable per diem rate. The contractor shall retain supporting documentation for per diem paid to employees as evidence of actual payments, as required by the FAR 52.216-7 “Allowable Cost and Payment” clause of the contract.
N66001-11-C-4188
Page 28 of 40
(d) Transportation
(1) The contractor shall be paid on the basis of actual amounts paid to the extent that such transportation is necessary for the performance of work under the contract and is authorized in writing by the Government per paragraph (a).
(2) The contractor agrees, in the performance of necessary travel, to use the lowest cost mode commensurate with the requirements of the mission and in accordance with good traffic management principles. When it is necessary to use air or rail travel, the contractor agrees to use coach, tourist class or similar accommodations to the extent consistent with the successful and economical accomplishment of the mission for which the travel is being performed. Documentation must be provided to substantiate non-availability of coach or tourist if business or first class is proposed to accomplish travel requirements.
(3) When transportation by privately owned conveyance (POC) is authorized, the contractor shall be paid on a mileage basis not to exceed the applicable Government transportation rate specified in the travel regulations cited in FAR 31.205-46(a)(2) and is authorized in writing by the Government per paragraph (a).
(4) When transportation by privately owned (motor) vehicle (POV) is authorized, required travel of contractor personnel, that is not commuting travel, may be paid to the extent that it exceeds the normal commuting mileage of such employee. When an employee’s POV is used for travel between an employee’s residence or the Permanent Duty Station and one or more alternate work sites within the local area, the employee shall be paid mileage for the distance that exceeds the employee’s commuting distance.
(5) When transportation by a rental automobile, other special conveyance or public conveyance is authorized, the contractor shall be paid the rental and/or hiring charge and operating expenses incurred on official business (if not included in the rental or hiring charge). When the operating expenses are included in the rental or hiring charge, there should be a record of those expenses available to submit with the receipt. Examples of such operating expenses include: hiring charge (bus, streetcar or subway fares), gasoline and oil, parking, and tunnel tolls.
(6) Definitions:
(i) “Permanent Duty Station” (PDS) is the location of the employee’s permanent work assignment (i.e., the building or other place where the employee regularly reports for work.
(ii) “Privately Owned Conveyance” (POC) is any transportation mode used for the movement of persons from place to place, other than a Government conveyance or common carrier, including a conveyance loaned for a charge to, or rented at personal expense by, an employee for transportation while on travel when such rental conveyance has not been authorized/approved as a Special Conveyance.
(iii) “Privately Owned (Motor) Vehicle (POV)” is any motor vehicle (including an automobile, light truck, van or pickup truck) owned by, or on a long-term lease (12 or more months) to, an employee or that employee’s dependent for the primary purpose of providing personal transportation, that:
(a) is self-propelled and licensed to travel on the public highways;
(b) is designed to carry passengers or goods; and
(c) has four or more wheels or is a motorcycle or moped.
(iv) “Special Conveyance” is commercially rented or hired vehicles other than a POC and other than those owned or under contract to an agency.
(v) “Public Conveyance” is local public transportation (e.g., bus, streetcar, subway, etc) or taxicab.
(iv) “Residence” is the fixed or permanent domicile of a person that can be reasonably justified as a bona fide residence.
N66001-11-C-4188
Page 29 of 40
EXAMPLE 1: Employee’s one way commuting distance to regular place of work is 7 miles. Employee drives from residence to an alternate work site, a distance of 18 miles. Upon completion of work, employee returns to residence, a distance of 18 miles.
In this case, the employee is entitled to be reimbursed for the distance that exceeds the normal round trip commuting distance (14 miles). The employee is reimbursed for 22 miles (18 + 18 - 14 = 22).
EXAMPLE 2: Employee’s one way commuting distance to regular place of work is 15 miles. Employee drives from residence to an alternate work site, a distance of 5 miles. Upon completion of work, employee returns to residence, a distance of 5 miles.
In this case, the employee is not entitled to be reimbursed for the travel performed (10 miles), since the distance traveled is less than the commuting distance (30 miles) to the regular place of work.
EXAMPLE 3: Employee’s one way commuting distance to regular place of work is 15 miles. Employee drives to regular place of work. Employee is required to travel to an alternate work site, a distance of 30 miles. Upon completion of work, employee returns to residence, a distance of 15 miles.
In this case, the employee is entitled to be reimbursed for the distance that exceeds the normal round trip commuting distance (30 miles). The employee is reimbursed for 30 miles (15 + 30 + 15 - 30 = 30).
EXAMPLE 4: Employee’s one way commuting distance to regular place of work is 12 miles. In the morning the employee drives to an alternate work site (45 miles). In the afternoon the employee returns to the regular place of work (67 miles). After completion of work, employee returns to residence, a distance of 12 miles.
In this case, the employee is entitled to be reimbursed for the distance that exceeds the normal round trip commuting distance (24 miles). The employee is reimbursed for 100 miles (45 + 67 + 12 - 24 = 100).
EXAMPLE 5: Employee’s one way commuting distance to regular place of work is 35 miles. Employee drives to the regular place of work (35 miles). Later, the employee drives to alternate work site #1 (50 miles) and then to alternate work site #2 (25 miles). Employee then drives to residence (10 miles).
In this case, the employee is entitled to be reimbursed for the distance that exceeds the normal commuting distance (70 miles). The employee is reimbursed for 50 miles (35 + 50 + 25 + 10 - 70 = 50).
EXAMPLE 6: Employee’s one way commuting distance to regular place of work is 20 miles. Employee drives to the regular place of work (20 miles). Later, the employee drives to alternate work site #1 (10 miles) and then to alternate work site #2 (5 miles). Employee then drives to residence (2 miles).
In this case, the employee is not entitled to be reimbursed for the travel performed (37 miles), since the distance traveled is less than the commuting distance (40 miles) to the regular place of work.
N66001-11-C-4188
Page 30 of 40
Section I - Contract Clauses
CLAUSES INCORPORATED BY REFERENCE
| 52.202-1 | Definitions | JUL 2004 |
| 52.203-3 | Gratuities | APR 1984 |
| 52.203-5 | Covenant Against Contingent Fees | APR 1984 |
| 52.203-6 | Restrictions On Subcontractor Sales To The Government | SEP 2006 |
| 52.203-7 | Anti-Kickback Procedures | OCT 2010 |
| 52.203-8 | Cancellation, Rescission, and Recovery of Funds for Illegal or JAN 1997 Improper Activity |
|
| 52.203-10 | Price Or Fee Adjustment For Illegal Or Improper Activity | JAN 1997 |
| 52.203-12 | Limitation On Payments To Influence Certain Federal Transactions | OCT 2010 |
| 52.204-4 | Printed or Copied Double-Sided on Postconsumer Fiber Content Paper | MAY 2011 |
| 52.204-7 | Central Contractor Registration | APR 2008 |
| 52.209-6 | Protecting the Government's Interest When Subcontracting DEC 2010 | |
| With Contractors Debarred, Suspended, or Proposed for Debarment | ||
| 52.215-2 | Audit and Records--Negotiation | OCT 2010 |
| 52.215-8 | Order of Precedence--Uniform Contract Format | OCT 1997 |
| 52.215-15 | Pension Adjustments and Asset Reversions | OCT 2010 |
| 52.215-17 | Waiver of Facilities Capital Cost of Money | OCT 1997 |
| 52.215-18 | Reversion or Adjustment of Plans for Postretirement Benefits JUL 2005 (PRB) Other than Pensions |
|
| 52.215-19 | Notification of Ownership Changes | OCT 1997 |
| 52.215-20 Alt II | Requirements for Cost or Pricing Data or Information Other OCT 1997 Than Cost or Pricing Data (Oct 2010) - Alternate II |
|
| 52.219-28 | Post-Award Small Business Program Rerepresentation | APR 2009 |
| 52.222-3 | Convict Labor | JUN 2003 |
| 52.222-21 | Prohibition Of Segregated Facilities | FEB 1999 |
| 52.222-26 | Equal Opportunity | MAR 2007 |
| 52.222-35 | Equal Opportunity for Veterans | SEP 2010 |
| 52.222-36 | Affirmative Action For Workers With Disabilities | OCT 2010 |
| 52.222-37 | Employment Reports on Veterans | SEP 2010 |
| 52.222-40 | Notification of Employee Rights Under the National Labor DEC 2010 Relations Act | |
| 52.222-50 | Combating Trafficking in Persons | FEB 2009 |
| 52.222-54 | Employment Eligibility Verification | JAN 2009 |
| 52.223-6 | Drug-Free Workplace | MAY 2001 |
| 52.223-18 | Encouraging Contractor Policies To Ban Text Messaging While Driving | AUG 2011 |
| 52.225-13 | Restrictions on Certain Foreign Purchases | JUN 2008 |
| 52.227-1 | Authorization and Consent | DEC 2007 |
| 52.227-1 Alt I | Authorization And Consent (Dec 2007) - Alternate I | APR 1984 |
| 52.227-2 | Notice And Assistance Regarding Patent And Copyright Infringement | DEC 2007 |
| 52.227-3 | Patent Indemnity | APR 1984 |
| 52.227-11 | Patent Rights--Ownership By The Contractor | DEC 2007 |
| 52.228-5 | Insurance - Work On A Government Installation | JAN 1997 |
| 52.228-7 | Insurance--Liability To Third Persons | MAR 1996 |
| 52.230-3 | Disclosure And Consistency Of Cost Accounting Practices | OCT 2008 |
N66001-11-C-4188
Page 31 of 40
| 52.232-2 | Payments Under Fixed-Price Research And Development Contracts | APR 1984 |
| 52.232-8 | Discounts For Prompt Payment | FEB 2002 |
| 52.232-9 | Limitation On Withholding Of Payments | APR 1984 |
| 52.232-17 | Interest | OCT 2010 |
| 52.232-23 | Assignment Of Claims | JAN 1986 |
| 52.232-23 Alt I | Assignment of Claims (Jan 1986) - Alternate I | APR 1984 |
| 52.232-25 | Prompt Payment | OCT 2008 |
| 52.232-25 Alt I | Prompt Payment (Oct 2008) Alternate I | FEB 2002 |
| 52.232-33 | Payment by Electronic Funds Transfer--Central Contractor OCT 2003 Registration | |
| 52.233-1 | Disputes | JUL 2002 |
| 52.233-3 | Protest After Award | AUG 1996 |
| 52.233-3 Alt I | Protest After Award (Aug 1996) - Alternate I | JUN 1985 |
| 52.233-4 | Applicable Law for Breach of Contract Claim | OCT 2004 |
| 52.237-2 | Protection Of Government Buildings, Equipment, And Vegetation | APR 1984 |
| 52.242-13 | Bankruptcy | JUL 1995 |
| 52.243-1 Alt I | Changes--Fixed Price (Aug 1987) - Alternate I | APR 1984 |
| 52.243-1 Alt V | Changes--Fixed-Price (Aug 1987) - Alternate V | APR 1984 |
| 52.244-6 | Subcontracts for Commercial Items | DEC 2010 |
| 52.245-1 | Government Property | AUG 2010 |
| 52.245-9 | Use And Charges | AUG 2010 |
| 52.246-25 | Limitation Of Liability--Services | FEB 1997 |
| 52.247-63 | Preference For U.S. Flag Air Carriers | JUN 2003 |
| 52.249-2 | Termination For Convenience Of The Government (Fixed- MAY 2004 Price) | |
| 52.249-9 | Default (Fixed-Priced Research And Development) | APR 1984 |
| 52.253-1 | Computer Generated Forms | JAN 1991 |
| 252.201-7000 | Contracting Officer's Representative | DEC 1991 |
| 252.203-7000 | Requirements Relating to Compensation of Former DoD Officials | JAN 2009 |
| 252.203-7001 | Prohibition On Persons Convicted of Fraud or Other Defense- DEC 2008 Contract-Related Felonies | |
| 252.203-7002 | Requirement to Inform Employees of Whistleblower Rights JAN 2009 | |
| 252.203-7002 | Requirement to Inform Employees of Whistleblower Rights JAN 2009 | |
| 252.204-7003 | Control Of Government Personnel Work Product | APR 1992 |
| 252.204-7004 Alt A C | entral Contractor Registration (52.204-7) Alternate A | SEP 2007 |
| 252.204-7006 | Billing Instructions | OCT 2005 |
| 252.209-7004 | Subcontracting With Firms That Are Owned or Controlled By DEC 2006 The Government of a Terrorist Country | |
| 252.223-7006 | Prohibition On Storage And Disposal Of Toxic And Hazardous Materials | APR 1993 |
| 252.225-7012 | Preference For Certain Domestic Commodities | JUN 2010 |
| 252.226-7001 | Utilization of Indian Organizations and Indian-Owned Economic Enterprises, and Native Hawaiian Small Business Concerns | SEP 2004 |
| 252.227-7012 | Patent License And Release Contract | SEP 1999 |
| 252.227-7013 | Rights in Technical Data--Noncommercial Items | MAR 2011 |
| 252.227-7014 | Rights in Noncommercial Computer Software and Noncommercial Computer Software Documentation | MAR 2011 |
| 252.227-7015 | Technical Data--Commercial Items | MAR 2011 |
| 252.227-7016 | Rights in Bid or Proposal Information | JAN 2011 |
| 252.227-7019 | Validation of Asserted Restrictions--Computer Software | JUN 1995 |
| 252.227-7027 | Deferred Ordering Of Technical Data Or Computer Software APR 1988 |
N66001-11-C-4188
Page 32 of 40
| 252.227-7030 | Technical Data--Withholding Of Payment | MAR 2000 |
| 252.227-7037 | Validation of Restrictive Markings on Technical Data | SEP 1999 |
| 252.231-7000 | Supplemental Cost Principles | DEC 1991 |
| 252.232-7010 | Levies on Contract Payments | DEC 2006 |
| 252.235-7011 | Final Scientific or Technical Report | NOV 2004 |
| 252.242-7004 | Material Management And Accounting System | MAY 2011 |
| 252.243-7001 | Pricing Of Contract Modifications | DEC 1991 |
| 252.243-7002 | Requests for Equitable Adjustment | MAR 1998 |
| 252.244-7000 | Subcontracts for Commercial Items and Commercial | NOV 2010 |
| Components (DoD Contracts) | ||
| 252.246-7000 | Material Inspection And Receiving Report | MAR 2008 |
CLAUSES INCORPORATED BY FULL TEXT
52.215-19 NOTIFICATION OF OWNERSHIP CHANGES (OCT 1997)
(a) The Contractor shall make the following notifications in writing:
(1) When the Contractor becomes aware that a change in its ownership has occurred, or is certain to occur, that could result in changes in the valuation of its capitalized assets in the accounting records, the Contractor shall notify the Administrative Contracting Officer (ACO) within 30 days.
(2) The Contractor shall also notify the ACO within 30 days whenever changes to asset valuations or any other cost changes have occurred or are certain to occur as a result of a change in ownership.
(b) The Contractor shall--
(1) Maintain current, accurate, and complete inventory records of assets and their costs;
(2) Provide the ACO or designated representative ready access to the records upon request;
(3) Ensure that all individual and grouped assets, their capitalized values, accumulated depreciation or amortization, and remaining useful lives are identified accurately before and after each of the Contractor's ownership changes; and
(4) Retain and continue to maintain depreciation and amortization schedules based on the asset records maintained before each Contractor ownership change.
The Contractor shall include the substance of this clause in all subcontracts under this contract that meet the applicability requirement of FAR 15.408(k).
(End of clause)
52.217-9 OPTION TO EXTEND THE TERM OF THE CONTRACT (MAR 2000)
(a) The Government may extend the term of this contract by written notice to the Contractor within the period of performance of this contract; provided that the Government gives the Contractor a preliminary written notice of its intent to extend at least 30 days before the contract expires. The preliminary notice does not commit the Government to an extension.
(b) If the Government exercises this option, the extended contract shall be considered to include this option clause.
N66001-11-C-4188
Page 33 of 40
(c) The total duration of this contract, including the exercise of any options under this clause, shall not exceed five years.
(End of clause)
52.232-32 PERFORMANCE-BASED PAYMENTS (AUG 2010)
(a) Amount of payments and limitations on payments. Subject to such other limitations and conditions as are specified in this contract and this clause, the amount of payments and limitations on payments shall be specified in the contract's description of the basis for payment.
(b) Contractor request for performance-based payment. The Contractor may submit requests for payment of performance-based payments not more frequently than monthly, in a form and manner acceptable to the Contracting Officer. Unless otherwise authorized by the Contracting Officer, all performance-based payments in any period for which payment is being requested shall be included in a single request, appropriately itemized and totaled. The Contractor's request shall contain the information and certification detailed in paragraphs (l) and (m) of this clause.
(c) Approval and payment of requests.
(1) The Contractor shall not be entitled to payment of a request for performance-based payment prior to successful accomplishment of the event or performance criterion for which payment is requested. The Contracting Officer shall determine whether the event or performance criterion for which payment is requested has been successfully accomplished in accordance with the terms of the contract. The Contracting Officer may, at any time, require the Contractor to substantiate the successful performance of any event or performance criterion which has been or is represented as being payable.
(2) A payment under this performance-based payment clause is a contract financing payment under the Prompt Payment clause of this contract and not subject to the interest penalty provisions of the Prompt Payment Act. The designated payment office will pay approved requests on the 30th day after receipt of the request for performance-based payment by the designated payment office. However, the designated payment office is not required to provide payment if the Contracting Officer requires substantiation as provided in paragraph (c)(1) of this clause, or inquires into the status of an event or performance criterion, or into any of the conditions listed in paragraph (e) of this clause, or into the Contractor certification. The payment period will not begin until the Contracting Officer approves the request.
(3) The approval by the Contracting Officer of a request for performance-based payment does not constitute an acceptance by the Government and does not excuse the Contractor from performance of obligations under this contract.
(d) Liquidation of performance-based payments.
(1) Performance-based finance amounts paid prior to payment for delivery of an item shall be liquidated by deducting a percentage or a designated dollar amount from the delivery payment. If the performance-based finance payments are on a delivery item basis, the liquidation amount for each such line item shall be the percent of that delivery item price that was previously paid under performance-based finance payments or the designated dollar amount. If the performance-based finance payments are on a whole contract basis, liquidation shall be by either predesignated liquidation amounts or a liquidation percentage.
(2) If at any time the amount of payments under this contract exceeds any limitation in this contract, the Contractor shall repay to the Government the excess. Unless otherwise determined by the Contracting Officer, such excess shall be credited as a reduction in the unliquidated performance-based payment balance(s), after adjustment of invoice payments and balances for any retroactive price adjustments.
N66001-11-C-4188
Page 34 of 40
(e) Reduction or suspension of performance-based payments. The Contracting Officer may reduce or suspend performance-based payments, liquidate performance-based payments by deduction from any payment under the contract, or take a combination of these actions after finding upon substantial evidence any of the following conditions:
(1) The Contractor failed to comply with any material requirement of this contract (which includes paragraphs (h) and (i) of this clause).
(2) Performance of this contract is endangered by the Contractor's --
(i) Failure to make progress; or
(ii) Unsatisfactory financial condition.
(3) The Contractor is delinquent in payment of any subcontractor or supplier under this contract in the ordinary course of business.
(f) Title.
(1) Title to the property described in this paragraph (f) shall vest in the Government. Vestiture shall be immediately upon the date of the first performance-based payment under this contract, for property acquired or produced before that date. Otherwise, vestiture shall occur when the property is or should have been allocable or properly chargeable to this contract
(2) "Property," as used in this clause, includes all of the following described items acquired or produced by the Contractor that are or should be allocable or properly chargeable to this contract under sound and generally accepted accounting principles and practices:
(i) Parts, materials, inventories, and work in process;
(ii) Special tooling and special test equipment to which the Government is to acquire title;
(iii) Nondurable (i.e., noncapital) tools, jigs, dies, fixtures, molds, patterns, taps, gauges, test equipment and other similar manufacturing aids, title to which would not be obtained as special tooling under subparagraph (f)(2)(ii) of this clause; and
(iv) Drawings and technical data, to the extent the Contractor or subcontractors are required to deliver them to the Government by other clauses of this contract.
(3) Although title to property is in the Government under this clause, other applicable clauses of this contract (e.g., the termination or clauses) shall determine the handling and disposition of the property.
(4) The Contractor may sell any scrap resulting from production under this contract, without requesting the Contracting Officer's approval, provided that any significant reduction in the value of the property to which the Government has title under this clause is reported in writing to the Contracting Officer.
(5) In order to acquire for its own use or dispose of property to which title is vested in the Government under this clause, the Contractor shall obtain the Contracting Officer's advance approval of the action and the terms. If approved, the basis for payment (the events or performance criteria) to which the property is related shall be deemed to be not in compliance with the terms of the contract and not payable (if the property is part of or needed for performance), and the Contractor shall refund the related performance-based payments in accordance with paragraph (d) of this clause.
(6) When the Contractor completes all of the obligations under this contract, including liquidation of all performance-based payments, title shall vest in the Contractor for all property (or the proceeds thereof) not --
N66001-11-C-4188
Page 35 of 40
(i) Delivered to, and accepted by, the Government under this contract; or
(ii) Incorporated in supplies delivered to, and accepted by, the Government under this contract and to which title is vested in the Government under this clause.
(7) The terms of this contract concerning liability for Government-furnished property shall not apply to property to which the Government acquired title solely under this clause.
(g) Risk of loss. Before delivery to and acceptance by the Government, the Contractor shall bear the risk of loss for property, the title to which vests in the Government under this clause, except to the extent the Government expressly assumes the risk. If any property is lost, stolen, damaged or destroyed, the basis of payment (the events or performance criteria) to which the property is related shall be deemed to be not in compliance with the terms of the contract and not payable (if the property is part of or needed for performance), and the Contractor shall refund the related performance-based payments in accordance with paragraph (d) of this clause.
(h) Records and controls. The Contractor shall maintain records and controls adequate for administration of this clause. The Contractor shall have no entitlement to performance-based payments during any time the Contractor's records or controls are determined by the Contracting Officer to be inadequate for administration of this clause.
(i) Reports and Government access. The Contractor shall promptly furnish reports, certificates, financial statements, and other pertinent information requested by the Contracting Officer for the administration of this clause and to determine that an event or other criterion prompting a financing payment has been successfully accomplished. The Contractor shall give the Government reasonable opportunity to examine and verify the Contractor's records and to examine and verify the Contractor's performance of this contract for administration of this clause.
(j) Special terms regarding default. If this contract is terminated under the Default clause,
(1) the Contractor shall, on demand, repay to the Government the amount of unliquidated performance-based payments, and
(2) title shall vest in the Contractor, on full liquidation of all performance-based payments, for all property for which the Government elects not to require delivery under the Default clause of this contract. The Government shall be liable for no payment except as provided by the Default clause.
(k) Reservation of rights.
(1) No payment or vesting of title under this clause shall --
(i) Excuse the Contractor from performance of obligations under this contract; or
(ii) Constitute a waiver of any of the rights or remedies of the parties under the contract.
(2) The Government's rights and remedies under this clause --
(i) Shall not be exclusive, but rather shall be in addition to any other rights and remedies provided by law or this contract; and
(ii) Shall not be affected by delayed, partial, or omitted exercise of any right, remedy, power, or privilege, nor shall such exercise or any single exercise preclude or impair any further exercise under this clause or the exercise of any other right, power, or privilege of the Government.
(l) Content of Contractor's request for performance-based payment. The Contractor's request for performance-based payment shall contain the following:
N66001-11-C-4188
Page 36 of 40
(1) The name and address of the Contractor;
(2) The date of the request for performance-based payment;
(3) The contract number and/or other identifier of the contract or order under which the request is made;
(4) Such information and documentation as is required by the contract's description of the basis for payment; and
(5) A certification by a Contractor official authorized to bind the Contractor, as specified in paragraph (m) of this clause.
(m) Content of Contractor's certification. As required in paragraph (l)(5) of this clause, the Contractor shall make the following certification in each request for performance-based payment:
I certify to the best of my knowledge and belief that --
(1) This request for performance-based payment is true and correct; this request (and attachments) has been prepared from the books and records of the Contractor, in accordance with the contract and the instructions of the Contracting Officer;
(2) (Except as reported in writing on_________ ), all payments to subcontractors and suppliers under this contract have been paid, or will be paid, currently, when due in the ordinary course of business;
(3) There are no encumbrances (except as reported in writing on_______ ) against the property acquired or produced for, and allocated or properly chargeable to, the contract which would affect or impair the Government's title;
(4) There has been no materially adverse change in the financial condition of the Contractor since the submission by
the Contractor to the Government of the most recent written information dated____________ ; and
(5) After the making of this requested performance-based payment, the amount of all payments for each deliverable item for which performance-based payments have been requested will not exceed any limitation in the contract, and the amount of all payments under the contract will not exceed any limitation in the contract.
(End of Clause)
52.252-2 CLAUSES INCORPORATED BY REFERENCE (FEB 1998)
This contract incorporates one or more clauses by reference, with the same force and effect as if they were given in full text. Upon request, the Contracting Officer will make their full text available. Also, the full text of a clause may be accessed electronically at this/these address(es):
http://farsite.hill.af.mil
http://www.acquisition.gov
(End of clause)
52.252-6 AUTHORIZED DEVIATIONS IN CLAUSES (APR 1984)
(a) The use in this solicitation or contract of any Federal Acquisition Regulation (48 CFR Chapter 1) clause with an authorized deviation is indicated by the addition of "(DEVIATION)" after the date of the clause.
N66001-11-C-4188
Page 37 of 40
(b) The use in this solicitation or contract of any Defense Federal Acquisition Regulation Supplement (48 CFR Chapter 2) clause with an authorized deviation is indicated by the addition of "(DEVIATION)" after the name of the regulation.
(End of clause)
252.204-7008 EXPORT-CONTROLLED ITEMS (APR 2010)
(a) Definition. Export-controlled items, as used in this clause, means items subject to the Export Administration Regulations (EAR) (15 CFR parts 730-774) or the International Traffic in Arms Regulations (ITAR) (22 CFR parts 120-130). The term includes:
(1) Defense items, defined in the Arms Export Control Act, 22 U.S.C. 2778(j)(4)(A), as defense articles, defense services, and related technical data, and further defined in the ITAR, 22 CFR part 120.
(2) Items, defined in the EAR as ̏commodities, software, and technology," terms that are also defined in the EAR, 15 CFR 772.1.
(b) The Contractor shall comply with all applicable laws and regulations regarding export-controlled items, including, but not limited to, the requirement for Contractors to register with the Department of State in accordance with the ITAR. The Contractor shall consult with the Department of State regarding any questions relating to compliance with the ITAR and shall consult with the Department of Commerce regarding any questions relating to compliance with the EAR.
(c) The Contractor's responsibility to comply with all applicable laws and regulations regarding export-controlled items exists independent of, and is not established or limited by, the information provided by this clause.
(d) Nothing in the terms of this contract adds to, changes, supersedes, or waives any of the requirements of applicable Federal laws, Executive orders, and regulations, including but not limited to--
(1) The Export Administration Act of 1979, as amended (50 U.S.C. App. 2401, et seq.);
(2) The Arms Export Control Act (22 U.S.C. 2751, et seq.);
(3) The International Emergency Economic Powers Act (50 U.S.C. 1701, et seq.);
(4) The Export Administration Regulations (15 CFR parts 730-774);
(5) The International Traffic in Arms Regulations (22 CFR parts 120-130); and
(6) Executive Order 13222, as extended.
(e) The Contractor shall include the substance of this clause, including this paragraph (e), in all subcontracts. (End of clause)
N66001-11-C-4188
Page 38 of 40
252.232-7003 ELECTRONIC SUBMISSION OF PAYMENT REQUESTS AND RECEIVING REPORTS (MAR 2008)
(a) Definitions. As used in this clause--
(1) Contract financing payment and invoice payment have the meanings given in section 32.001 of the Federal Acquisition Regulation.
(2) Electronic form means any automated system that transmits information electronically from the initiating system to all affected systems. Facsimile, e-mail, and scanned documents are not acceptable electronic forms for submission of payment requests. However, scanned documents are acceptable when they are part of a submission of a payment request made using Wide Area WorkFlow (WAWF) or another electronic form authorized by the Contracting Officer.
(3) Payment request means any request for contract financing payment or invoice payment submitted by the Contractor under this contract.
(b) Except as provided in paragraph (c) of this clause, the Contractor shall submit payment requests and receiving reports using WAWF, in one of the following electronic formats that WAWF accepts: Electronic Data Interchange, Secure File Transfer Protocol, or World Wide Web input. Information regarding WAWF is available on the Internet at https://wawf.eb.mil/.
(c) The Contractor may submit a payment request and receiving report using other than WAWF only when--
(1) The Contracting Officer authorizes use of another electronic form. With such an authorization, the Contractor and the Contracting Officer shall agree to a plan, which shall include a timeline, specifying when the Contractor will transfer to WAWF;
(2) DoD is unable to receive a payment request or provide acceptance in electronic form;
(3) The Contracting Officer administering the contract for payment has determined, in writing, that electronic submission would be unduly burdensome to the Contractor. In such cases, the Contractor shall include a copy of the Contracting Officer's determination with each request for payment; or
(4) DoD makes payment for commercial transportation services provided under a Government rate tender or a contract for transportation services using a DoD-approved electronic third party payment system or other exempted vendor payment/invoicing system (e.g., PowerTrack, Transportation Financial Management System, and Cargo and Billing System).
(d) The Contractor shall submit any non-electronic payment requests using the method or methods specified in Section G of the contract.
(e) In addition to the requirements of this clause, the Contractor shall meet the requirements of the appropriate payment clauses in this contract when submitting payments requests.
(End of clause)
252.247-7024 Notification of Transportation of Supplies by Sea (MAR 2000)
(a) The Contractor has indicated by the response to the solicitation provision, Representation of Extent of Transportation by Sea, that it did not anticipate transporting by sea any supplies. If, however, after the award of this contract, the Contractor learns that supplies, as defined in the Transportation of Supplies by Sea clause of this contract, will be transported by sea, the Contractor --
(1) Shall notify the Contracting Officer of that fact; and
N66001-11-C-4188
Page 39 of 40
(2) Hereby agrees to comply with all the terms and conditions of the Transportation of Supplies by Sea clause of this contract.
(b) The Contractor shall include this clause; including this paragraph (b), revised as necessary to reflect the relationship of the contracting parties--
(1) In all subcontracts under this contract, if this contract is a construction contract; or
(2) If this contract is not a construction contract, in all subcontracts under this contract that are for--
(i) Noncommercial items; or
(ii) Commercial items that--
(A) The Contractor is reselling or distributing to the Government without adding value (generally, the Contractor does not add value to items that it subcontracts for f.o.b. destination shipment);
(B) Are shipped in direct support of U.S. military contingency operations, exercises, or forces deployed in humanitarian or peacekeeping operations; or
(C) Are commissary or exchange cargoes transported outside of the Defense Transportation System in accordance with 10 U.S.C. 2643.
(End of clause)
N66001-11-C-4188
Page 40 of 40
Section J - List of Documents, Exhibits and Other Attachments
Exhibit/Attachment Table of Contents
| DOCUMENT TYPE | DESCRIPTION | PAGES | DATE |
| Exhibit A | Base Period CDRLs | 4 | 26-SEP-2011 |
| Exhibit B | Option I CDRLs | 4 | 26-SEP-2011 |
| Exhibit C | Option II CDRLs | 4 | 26-SEP-2011 |
| Exhibit D | Option III CDRLs | 4 | 26-SEP-2011 |
| Exhibit E | Option IV CDRLs | 4 | 26-SEP-2011 |
| Attachment 1 | Performance Based | 5 | 26-SEP-2011 |
| Payments Schedule | |||
| Attachment 2 | Clause 252.227-7017 | 2 | 28-SEP-2011 |
AMENDMENT OF SOLITICATION/MODIFICATION OF CONTRACT

| 1 |
SECTION SF 30 BLOCK 14 CONTINUATION PAGE
SUMMARY OF CHANGES
SECTION C - DESCRIPTIONS AND SPECIFICATIONS
The following have been modified: STATEMENT OF WORK (SOW)
Aethlon Medical, Inc.
DATE: 11 December 2013, Revised: 12 March 2015
TITLE: Broad Spectrum Countermeasures for Viral and Bacterial Sepsis using Dialysis-Like Devices
| 1.0 | Scope |
The scope of this effort is to use Aethlon’s Hemopurifier as the core technology within an extracorporeal blood purification device that would simultaneously remove: viruses, virally-derived immunosuppressive glycoproteins, and multiple classes of exosomes; complement activation, activation of virus growth (e.g. cytomegalovirus) and TLR activation, all of which have implications to the promotion of the well-being and recovery of wounded warfighters and the prevention of sepsis.
| 1.1 | Introduction |
This effort will create an adaptable dialysis-like platform (ADAPT) technology that allows for the selective removal of harmful agents from the entire circulatory system. This revolutionary advance overcomes the limitation of devices that indiscriminately adsorb or solely capture particles by molecule size. The platform will provide an expansive therapeutic filtration mechanism to immobilize multiple affinity agents directed toward precursors to sepsis, bacterial toxins, viral pathogens, and disease enhancing particles transported by exosomes. To insure benefit to wounded warfighters, this effort will advance an innovative strategy that will allow therapy administration without systemic anticoagulation.
The ADAPT platform has been shown previously to create a broad-spectrum antiviral device that immobilized one lectin affinity agent, resulting in the effective capture of all tested Category A pathogens, as well as exosomes underlying tuberculosis and cancer. In human studies, this same device, known as the Hemopurifier, consistently provided greater than 50% average viral load reductions during four-hour treatment periods in both hepatitis-C and HIV infected individuals without antiviral drug therapy.
The resulting device would save thousands of military and civilian lives each year. Each of these technology advancements will be integrated into a single cartridge that will provide decision-free and life-saving medical care for the wounded warfighter.
| 1.2 | Background |
The goal of the DLT program is to develop a portable device that removes “dirty” blood from the body, separates harmful agents, and returns “clean” blood to the body in a manner similar to dialysis treatment of kidney failure. While the device could have an impact across multiple areas of medicine, the target application for this device is sepsis. The envisioned device will persistently interrogate the entire blood volume, providing early identification of the presence of a pathogen. Once the presence of pathogens has been confirmed, the DLT device will provide continuous "label-free" removal of pathogens, toxins and activated patient cells without pathogen identification or use of pathogen-specific binding chemistries. As a final step in the treatment process, the DLT device will enable closed-loop therapy based on continuous, reduced dimensionality modeling of patient health. Predictive modeling in this fashion will allow us to identify sepsis early, learn what we need to remove, and direct the most effective intervention to improve patient health. This cycle of sensing, adjustment, estimation, computation, and manipulation will modulate key health parameters faster than the underlying disease process and drive the patient towards a stable, healthy state.
| 2 |
| 2.0 | Technical Requirements |
| 2.1 | Human and Animal use |
Human use is anticipated in this effort, specifically related to the use of human blood. The grantee shall obtain all necessary Institutional Review Board (IRB) approvals, show proper assurance documentation, and obtain proper approval from the Government officials prior to human use testing. Funds associated with human subjected testing shall not be released until IRB documentation has been provided to SSC’s HRPO and approval to release funds has been obtained.
Animal use is anticipated in this effort. The contractor shall obtain all necessary Institutional Animal Care and Utilization Committee (IACUC) approval and demonstrate this approval to the Government (both ACURO and SSC-Pacific) prior to beginning experimentation with animals. If animal use is no longer anticipated, or changes significantly from the approved IACUC then the Principal Investigator (PI) must submit a letter stating the discontinuation of animal use for this effort and/or receive appropriate authorization for IACUC changes of previously specified protocols. Unless prior approval by DARPA is given IACUC documentation must be provided prior to contract award.
| 2.2 | Base Effort (Year 1) |
| 2.2.1 | Subtask 1a: Anticoagulant-free Hemopurification Device |
| 2.2.1.1 | Write requirements definition for the extracorporeal blood purification system and acquire necessary equipment. |
| 2.2.1.2 | Fabricate breadboard prototypes for anticoagulation-free anti-sepsis extracorporeal system (ASEPSYS) device. Fabricate prototype blood tubing sets. Acquire anti-thrombogenic surface-modified hollow fiber plasma separators. |
| 2.2.1.3 | Assemble and test breadboard ASEPSYS devices ex vivo with bovine blood. The test will most likely be conducted using a porcine model where the elapsed time to reach a pre-defined degree of clotting in the blood treatment device will be compared between the new device and two control groups; one using standard anticoagulant therapy and one using none. Determine contribution of the following techniques and approaches to eliminating anticoagulants: |
(1) Backflushing at regular intervals, (2) Turbo loop, (3) Continuous pre-dilution loop using re-captured hydration fluid, (4) Linear vs pulsatile blood flow, (5) Elimination of air/blood interfaces in extracorporeal circuit, (6) Anti-thrombogenic derivatized plasma separation membrane, and (7) Ultra-short half-life anticoagulant nafamostat mesilate.
| 2.2.1.4 | IRB Documentation Generation: The contractor shall obtain all necessary IRB documentation and obtain both institutional and Government (SSC-Pacific) approval in accordance with IRB documentation submission guidance prior to conducting human subject testing. |
Milestones
M1: Demonstrate the effectiveness of the prototype device in preventing platelet activation or clotting in at least a 2 hour blood pumping experiment at 100 mL/hr blood flow.
| 2.2.2 | Subtask 2: Removal of Sepsis Precursors |
| 2.2.2.1 | Begin to develop a device based on Aethlon’s ADAPT system to efficiently capture sepsis precursors identified as potentially important in killing patients undergoing sepsis. The strategy is takes advantage of the flexibility and rapidity of modification of our ADAPT platform system to test any sepsis precursor candidates that circulate in the blood. The sepsis precursors that will be targeted are shown in Table I, in order of importance. No test for the removal of bacterial toxins. No testing for removal of cytokines, since the evidence to date does not support a role for them in death due to sepsis. Additional factors may become known during the grant period and those will also be tested as time and budget permit. |
| 3 |
| 2.2.2.2 | Screening Capture Agents: Perform initial screening of the different proposed capture agents by measuring binding affinity and kinetics using surface plasmon resonance (SPR) or biolayer surface interferometry (BLI). |
| 2.2.2.3 | Perform quantitative real time PCR will also be used to measure viral load, and specific DNA or RNA targets. |
Milestones
M2: Target capture > 50% in 24 hours for at least 1 target in blood or blood components
Table I Potential Target Sepsis Precursors and Broad Spectrum Binding Agents
| Septic Exosomes | Removal Agent |
| INOS exosomes | GNA |
| Viruses Associated with Sepsis | |
| Dengue Fever virus | GNA |
| H1N1 Flu virus | GNA |
| Cytomegalovirus ** | GNA |
| Herpes viruses – e.g. HSV 1 | GNA |
| Poxviruses - e.g. Vaccina (model for small pox) | GNA |
| Activation of Innate Immunity | |
| LPS endotoxins | GNA |
| LTA endotoxins | GNA |
| 2.3 | Option 1 (Year 2) |
| 2.3.2 | Subtask 1a: Anticoagulant-free Hemopurification Device |
| 2.3.2.1 | Demonstrate the effectiveness of the prototype device in vivo in animals preventing platelet activation or clotting in at least a 2 hour blood pumping experiment at 75 mL/min blood flow. |
| 2.3.2.2 | Formulate initial design based on work from previous phase. Begin to build and test selected instrument design and tubing sets. |
| 2.3.2.3 | Write and test software. Conduct ergonomic research. Begin discussions with System Integrator. |
| 2.3.2.4 | Complete fabrication and testing of prototype instrument. |
| 2.3.2.5 | Research literature and patent database for technical options for an on-line/in-line sensor with the ability to quantify the clearance of citrate and/or calcium in effluent dialysate. |
| 2.3.2.6 | Collect feasibility data on at least one sensor option. |
| 2.3.2.7 | Conduct a series of 21 experiments aimed at characterizing the contribution of several alternate fluidic designs and methods of perfusing plasma filters and affinity columns in the performance of affinity plasmapheresis. |
2,3.3 Subtask 2. Removal of Sepsis Precursors
| 2.3.3.1 | Build the ADAPT capture cartridges with the identified affinity agents. Measure the rate of capture of the specific targets from in ex vivo recirculation experiments from cell culture and blood. |
| 2.3.3.2 | Cartridge construction with optimized affinity matrix design for each potential target. Complete all capture agents screening. Initiate ex vivo capture studies from blood using the optimized cartridges. |
Milestones
M4: Target capture > 50% in 24 hours for at least 5 targets in blood or blood components.
M5: Milestone 5: Target capture > 90% in 24 hours for at least 3 targets in blood or blood components.
NOTE: TASK 2.3.2 SHALL NOT BE EXERCISED AND TASKING FUNDS RELEASED UNTIL IRB DOCUMENTATION AND PROPER IRB APPROVAL HAS BEEN OBTAINED.
| 4 |
| 2.4 | Option 2 (Year 3) |
| 2.4.1 | Subtask 1a: Anticoagulant-free Hemopurification Device |
| 2.4.1.1 | Design and fabricate optimized configuration(s) of hemopurification device(s) that contain(s) a combination of hemofilters, plasma filters, and affinity columns. |
Milestones
M6:
Define Aethlon’s GMP manufacturing process and revise and upgrade Aethlon’s quality procedures
and policies to the current state of the art. Demonstrate the safety of at least one prototype device within an optimized
fluidic circuit architecture preventing clotting in a 24 hour experiment in vivo at a blood flow rate of 200 ml/min using
either pigs or dogs.
| 2.4.2 | Subtask 4: Target Capture in Combined Agent Cartridge |
2.4.2.1 Evaluate contribution of manufacturing process variables to binding capacity of affinity resin.
2.4.2.2 Determine capacity requirements of affinity resin to multiple simultaneous targets.
2.4.2.3 Perform basic biocompatibility tests for the combination ADAPT device to confirm the combination cartridge does not present additional risk.
2.4.2.4 Finish construction and delivery of 25 experimental cartridges for testing by the system integrator.
Milestones
M7: Target capture > 90% in 24 hours (12 months) for at least 3 targets ex vivo in blood or blood components using the optimized cartridge.
M8: Pass biocompatibility tests for the combination ADAPT device.
M9: Construct and deliver of 25 prototype cartridges for testing by the system integrator.
| 2.5 | Option 3 (Year 4) |
| 2.5.1 | Subtask 1a: Anticoagulant-free Hemopurification Device |
| 2.5.1.1 | Complete Aethlon’s GMP procedure and establish
and maintain all GMP documentation for the company. |
Milestones
M11:
Develop a strategic plan for developing an alternate method of producing GNA by cloning the gene into
an alternate vector and identify potential partners for such production. Demonstrate the safety of an additional prototype
device within an optimized fluidic circuit architecture preventing clotting in a 24 hour experiment in vivo at a blood flow
rate of 200 ml/min using either pigs or dogs.
| 2.5.2 | Subtask 4: Target Capture in Combined Agent Cartridge |
2.5.2.2 Finish construction and delivery of 50 prototype cartridges for testing by the system integrator. The cartridges will need to be made available (packaged, labeled, sterilized and qualified) to the system integrator.
Milestones
M13: Construct and deliver of 50 prototype cartridges for testing by the system integrator.
2.6 Option 4 (Year 5)
2.6.1 Subtask 5: Testing of final product by System Integrator
2.6.1.1 System integrator acceptance of the hemofilter device.
| 2.6.1.3 | Prepare and submit IDE proposal for sepsis treatment. |
| 2.6.1.4 | Prepare and present Final report for DARPA. |
| 5 |
Milestones
M12: System Integrator approval of a sepsis precursor ADAPT treatment cartridge
| 3.0 | Program Management and Reviews |
| 3.1 | Program Management Plan |
The contractor shall develop a Program Management Plan. A graphical representation of this plan (Gantt chart is one example) identifying major tasks and their task leaders, milestones of the major task and their completion dates shall be generated. In addition, a graphical representation of budget shall be generated.
| 3.2 | Kick-off Meeting |
The contractor shall participate in a kick-off meeting within 60 days of contract award. The purpose of this meeting is to introduce key program personnel, discuss the proposed tasking, present the program schedule and milestones and the initial Program Management Plan.
| 3.3 | Quarterly Reviews |
The contractor shall hold quarterly reviews for the duration of this effort. The purpose of these reviews is to present a summary of work completed and milestones met, discuss any problems encountered, update the program schedule, present the program financial status, and discuss remaining work.
| 3.4 | Final Contract Review |
A final contract review held in place of the last quarterly review shall be hosted by the principal contractor. The purpose of this review is to present a summary of all work completed and milestones accomplished and to discuss any relevant future efforts similar to the contract that may be pursued.
4.0 Deliverables
The reports and presentation materials are to be delivered in accordance with the attached Data Delivery Matrix.
(End of SOW).
SECTION G - CONTRACT ADMINISTRATION DATA
| 6 |
The following have been modified:
SCHEDULE OF PAYMENT MILESTONES
12 month duration base period (Year 1)
| Milestone | Month | Payable Milestone | GOVT CONTRIBUTION |
| Subtask 1a | Anticoagulant-free Hemopurification Device | ($908,384) | |
| 2.2.1.1 | 1 | Write requirements definition for the extracorporeal blood purification system and acquire necessary equipment. | ($358,284) |
|
2.2.1.2 |
3 |
Fabricate breadboard prototypes for anticoagulation-free anti-sepsis extracorporeal system (ASEPSYS) device. Fabricate prototype blood tubing sets. Acquire anti- thrombogenic surface-modified hollow fiber plasma separators. |
($183,367) |
|
2.2.1.3 |
6 |
Assemble and test breadboard ASEPSYS devices. Evaluate the use of different techniques and approaches to eliminating anticoagulants. |
($183,367) |
|
2.2.1.4 |
12 |
Obtain all necessary IRB documentation and obtain both institutional and Government (SSC-Pacific) approval in accordance with IRB documentation submission guidance prior to conducting human or animal testing. |
($183,367) |
|
Subtask 2 & 4 |
Removal of Sepsis Precursors | ($1,066,663) | |
|
2.2.2.1 |
2 |
Begin to develop the Aethlon’s ADAPT device to efficiently capture sepsis precursors and acquire important equipment and supplies |
($416,424) |
|
2.2.2.2 |
5 |
Perform initial screening of the different proposed capture agents by measuring binding affinity and kinetics using surface plasmon resonance (SPR) or biolayer surface interferometry (BLI). |
($216,747) |
| 2.2.2.3 | 8 | Perform preliminary quantitative real time PCR to measure viral load, and specific DNA or RNA targets. | ($216,747) |
| M2 | 12 | Target capture > 50% in 24 hours for at least 1 target in blood or blood components | ($216,747) |
| Total | ($1,975,047) | ||
| 7 |
12 month duration option #1 period (Year2)
| Milestone | Month | Payable Milestone | GOVT CONTRIBUTION |
| Subtask 1a | Anticoagulant-free Hemopurification Device | ($782,322) | |
|
2.3.2.1 |
15 |
Demonstrate the effectiveness of the prototype device in vivo in animals preventing platelet activation or clotting in at least a 2 hour blood pumping experiment at 75 mL/min blood flow. |
($195,581) |
|
2.3.2.2 |
18 |
Formulate initial design based on work from previous phase. Begin to build and test selected instrument design and tubing sets. |
($195,581) |
|
2.3.2.3 |
21 |
Write and test software. Conduct ergonomic research. Begin discussions with System Integrator. |
($195,581) |
|
M3 |
24 |
Complete fabrication and testing of prototype instrument. Research literature and patent database for technical. |
($195,581) |
| Subtask 2 | Removal of Sepsis Precursors | ($835,124) | |
|
2.3.3.1 |
15 |
Build the ADAPT capture cartridges with the identified affinity agents. Measure the rate of capture of the specific targets from in ex vivo recirculation experiments from cell culture and blood. |
($208,781) |
| M4 | 18 | Target capture > 50% in 24 hours for at least 5 targets in blood or blood components. | ($208,781) |
|
2.3.3.2 |
21 |
Cartridge construction with optimized affinity matrix design for each potential target. Complete the capture agent screening. |
($208,781) |
| M5 | 24 | Target capture > 90% in 24 hours for at least 3 targets in blood or blood components. | ($208,781) |
| Total | ($1,617,446) | ||
| 8 |
12 month duration option #2 period (Year 3)
| Milestone | Month | Payable Milestone | GOVT CONTRIBUTION |
| Subtask 1a | Anticoagulant-free Hemopurification Device | ($372,328) | |
|
2.4.1.1 |
27 |
Design and fabricate optimized configuration(s) of hemopurification device(s) that contain(s) a combination of hemofilters, plasma filters, and affinity columns. |
($186,164) |
|
M6 |
36 |
Define Aethlon’s GMP manufacturing process and revise and upgrade Aethlon’s quality procedures and policies to the current state of the art. |
($186,164) |
| Subtask 2+4 | Target Capture in Combined Agent Cartridge | ($720,726) | |
|
2.4.2.1 |
27 |
Evaluate contribution of manufacturing process variables to binding capacity of affinity resin. |
($197,362) |
|
2.4.2.2 |
33 |
Determine capacity requirements of affinity resin to multiple simultaneous targets. |
($197,362) |
| 2.4.2.3 | 30 | Perform biocompatibility tests for the combination ADAPT device to confirm the combination cartridge does not present additional risk. | ($78,641) |
| 2.4.2.4 | 36 | Finish construction and delivery of 25 experimental cartridges for testing by the system integrator. | ($50,000) |
|
M9 |
36 |
Target capture > 90% in 24 hours (12 months) for at least 3 targets ex vivo in blood or blood components using the optimized cartridge. |
($197,361) |
| Total | ($1,093,054) | ||
| 9 |
12 month duration option #3 period (Year 4)
| Milestone | Month | Payable Milestone | GOVT CONTRIBUTION |
| Subtask 1a | Anticoagulant-free Hemopurification Device | ($276,172) | |
|
2.5.1.1 |
45 |
Complete Aethlon’s GMP procedure and establish and maintain all GMP documentation for the company. | ($90,008) |
|
M11 |
48 |
Develop a strategic plan for developing an alternate method of producing GNA by cloning the gene into an alternate vector and identify potential partners for such production. |
($186,164) |
| Subtask 2+4 | Target Capture in Combined Agent Cartridge | ($296,964) | |
| 2.5.2.2 | 48 | Finish construction and begin delivery of 50 prototype cartridges for testing by the system integrator. | ($296,964) |
| Total | ($573,136) | ||
| 10 |
12 month duration option #4 period (Year 5)
| Milestone | Month | Payable Milestone | GOVT CONTRIBUTION |
| Subtask 5 | Testing of final product by System Integrator | ($581,157) | |
| 2.6.1.1 | 51 | System integrator acceptance of the hemofilter device. | ($193,719) |
| 2.6.1.3 | 57 | Prepare and submit IDE proposal for sepsis treatment. | ($193,719) |
| 2.6.1.4 | 60 | Prepare and present Final Report for DARPA. | ($193,719) |
| Total | ($581,157) | ||
(End of Milestone Schedule)
(End of Summary of Changes)
| 11 |

| 1 |
SECTION SF 30 BLOCK 14 CONTINUATION PAGE
SUMMARY OF CHANGES
SECTION A - SOLICITATION/CONTRACT FORM
The standard size code 1,000 has been added.
The NAICS code 541711 has been added.
SECTION C - DESCRIPTIONS AND SPECIFICATIONS
The following have been modified:
STATEMENT OF WORK (SOW)
Aethlon Medical, Inc.
DATE: 12 March 2015; Modified: 15 July 2016
TITLE: Broad Spectrum Countermeasures for Viral and Bacterial Sepsis using Dialysis-Like Devices
| 1.0 | Scope |
The scope of this effort is to use Aethlon’s Hemopurifier as the core technology within an extracorporeal blood purification device that would simultaneously remove: viruses, virally-derived immunosuppressive glycoproteins, and multiple classes of exosomes; complement activation, activation of virus growth (e.g. cytomegalovirus) and TLR activation, all of which have implications to the promotion of the well-being and recovery of wounded warfighters and the prevention of sepsis.
| 1.1 | Introduction |
This effort will create an adaptable dialysis-like platform (ADAPT) technology that allows for the selective removal of harmful agents from the entire circulatory system. This revolutionary advance overcomes the limitation of devices that indiscriminately adsorb or solely capture particles by molecule size. The platform will provide an expansive therapeutic filtration mechanism to immobilize multiple affinity agents directed toward precursors to sepsis, bacterial toxins, viral pathogens, and disease enhancing particles transported by exosomes. To insure benefit to wounded warfighters, this effort will advance an innovative strategy that will allow therapy administration without systemic anticoagulation.
The ADAPT platform has been shown previously to create a broad-spectrum antiviral device that immobilized one lectin affinity agent, resulting in the effective capture of all tested Category A pathogens, as well as exosomes underlying tuberculosis and cancer. In human studies, this same device, known as the Hemopurifier, consistently provided greater than 50% average viral load reductions during four-hour treatment periods in both hepatitis-C and HIV infected individuals without antiviral drug therapy.
The resulting device would save thousands of military and civilian lives each year. Each of these technology advancements will be integrated into a single cartridge that will provide decision-free and life-saving medical care for the wounded warfighter.
| 2 |
| 1.2 | Background |
The goal of the DLT program is to develop a portable device that removes “dirty” blood from the body, separates harmful agents, and returns “clean” blood to the body in a manner similar to dialysis treatment of kidney failure. While the device could have an impact across multiple areas of medicine, the target application for this device is sepsis. The envisioned device will persistently interrogate the entire blood volume, providing early identification of the presence of a pathogen. Once the presence of pathogens has been confirmed, the DLT device will provide continuous "label-free" removal of pathogens, toxins and activated patient cells without pathogen identification or use of pathogen-specific binding chemistries. As a final step in the treatment process, the DLT device will enable closed-loop therapy based on continuous, reduced dimensionality modeling of patient health. Predictive modeling in this fashion will allow us to identify sepsis early, learn what we need to remove, and direct the most effective intervention to improve patient health. This cycle of sensing, adjustment, estimation, computation, and manipulation will modulate key health parameters faster than the underlying disease process and drive the patient towards a stable, healthy state.
| 2.0 | Technical Requirements |
| 2.1 | Human and Animal use |
Human use is anticipated in this effort, specifically related to the use of human blood. The grantee shall obtain all necessary Institutional Review Board (IRB) approvals, show proper assurance documentation, and obtain proper approval from the Government officials prior to human use testing. Funds associated with human subjected testing shall not be released until IRB documentation has been provided to SSC’s HRPO and approval to release funds has been obtained.
Animal use is anticipated in this effort. The contractor shall obtain all necessary Institutional Animal Care and Utilization Committee (IACUC) approval and demonstrate this approval to the Government (both ACURO and SSC-Pacific) prior to beginning experimentation with animals. If animal use is no longer anticipated, or changes significantly from the approved IACUC then the Principal Investigator (PI) must submit a letter stating the discontinuation of animal use for this effort and/or receive appropriate authorization for IACUC changes of previously specified protocols. Unless prior approval by DARPA is given IACUC documentation must be provided prior to contract award.
| 2.2 | Base Effort (Year 1) |
| 2.2.1 | Subtask 1a: Anticoagulant-free Hemopurification Device |
| 2.2.1.1 | Write requirements definition for the extracorporeal blood purification system and acquire necessary equipment. |
| 2.2.1.2 | Fabricate breadboard prototypes for anticoagulation-free anti-sepsis extracorporeal system (ASEPSYS) device. Fabricate prototype blood tubing sets. Acquire anti-thrombogenic surface-modified hollow fiber plasma separators. |
| 2.2.1.3 | Assemble and test breadboard ASEPSYS devices ex vivo with bovine blood. The test will most
likely be conducted using a porcine model where the elapsed time to reach a pre-defined degree of clotting in the blood treatment
device will be compared between the new device and two control groups; one using standard anticoagulant therapy and one using none.
Determine contribution of the following techniques and approaches to eliminating anticoagulants: (1) Backflushing at regular intervals, (2) Turbo loop, (3) Continuous pre-dilution loop using re-captured hydration fluid, (4) Linear vs pulsatile blood flow, (5) Elimination of air/blood interfaces in extracorporeal circuit, (6) Anti-thrombogenic derivatized plasma separation membrane, and (7) Ultra-short half-life anticoagulant nafamostat mesilate. |
| 2.2.1.4 | IRB Documentation Generation: The contractor shall obtain all necessary IRB documentation and obtain both institutional and Government (SSC-Pacific) approval in accordance with IRB documentation submission guidance prior to conducting human subject testing. |
Milestones
| M1: | Demonstrate the effectiveness of the prototype device in preventing platelet activation or clotting in at least a 2 hour blood pumping experiment at 100 mL/hr blood flow. |
| 2.2.2 | Subtask 2: Removal of Sepsis Precursors |
| 2.2.2.1 | Begin to develop a device based on Aethlon’s ADAPT system to efficiently capture sepsis precursors identified as potentially important in killing patients undergoing sepsis. The strategy is takes advantage of the flexibility and rapidity of modification of our ADAPT platform system to test any sepsis precursor candidates that circulate in the blood. The sepsis precursors that will be targeted are shown in Table I, in order of importance. No test for the removal of bacterial toxins. No testing for removal of cytokines, since the evidence to date does not support a role for them in death due to sepsis. Additional factors may become known during the grant period and those will also be tested as time and budget permit |
| 3 |
.
| 2.2.2.2 | Screening Capture Agents: Perform initial screening of the different proposed capture agents by measuring binding affinity and kinetics using surface plasmon resonance (SPR) or biolayer surface interferometry (BLI). |
| 2.2.2.3 | Perform quantitative real time PCR will also be used to measure viral load, and specific DNA or RNA targets. |
Milestones
M2: Target capture > 50% in 24 hours for at least 1 target in blood or blood components
Table I Potential Target Sepsis Precursors and Broad Spectrum Binding Agents
| Septic Exosomes | Removal Agent |
| INOS exosomes | GNA |
| Viruses Associated with Sepsis | |
| Dengue Fever virus | GNA |
| H1N1 Flu virus | GNA |
| Cytomegalovirus ** | GNA |
| Herpes viruses – e.g. HSV 1 | GNA |
| Poxviruses - e.g. Vaccina (model for small pox) | GNA |
| Activation of Innate Immunity | |
| LPS endotoxins | GNA |
| LTA endotoxins | GNA |
| 2.3 | Option 1 (Year 2) |
| 2.3.2 | Subtask 1a: Anticoagulant-free Hemopurification Device |
| 2.3.2.1 | Demonstrate the effectiveness of the prototype device in vivo in animals preventing platelet activation or clotting in at least a 2 hour blood pumping experiment at 75 mL/min blood flow. |
| 2.3.2.2 | Formulate initial design based on work from previous phase. Begin to build and test selected instrument design and tubing sets. |
| 2.3.2.3 | Write and test software. Conduct ergonomic research. Begin discussions with System Integrator. |
| 2.3.2.4 | Complete fabrication and testing of prototype instrument. |
| 2.3.2.5 | Research literature and patent database for technical options for an on-line/in-line sensor with the ability to quantify the clearance of citrate and/or calcium in effluent dialysate. |
| 2.3.2.6 | Collect feasibility data on at least one sensor option. |
| 2.3.2.7 | Conduct a series of 21 experiments aimed at characterizing the contribution of several alternate fluidic designs and methods of perfusing plasma filters and affinity columns in the performance of affinity plasmapheresis. |
2,3.3 Subtask 2. Removal of Sepsis Precursors
| 2.3.3.1 | Build the ADAPT capture cartridges with the identified affinity agents. Measure the rate of capture of the specific targets from in ex vivo recirculation experiments from cell culture and blood. |
| 2.3.3.2 | Cartridge construction with optimized affinity matrix design for each potential target. Complete all capture agents screening. Initiate ex vivo capture studies from blood using the optimized cartridges. |
Milestones
M4: Target capture > 50% in 24 hours for at least 5 targets in blood or blood components.
M5: Milestone 5: Target capture > 90% in 24 hours for at least 3 targets in blood or blood components.
NOTE: TASK 2.3.2 SHALL NOT BE EXERCISED AND TASKING FUNDS RELEASED UNTIL IRB DOCUMENTATION AND PROPER IRB APPROVAL HAS BEEN OBTAINED.
| 4 |
| 2.4 | Option 2 (Year 3) |
| 2.4.1 | Subtask 1a: Anticoagulant-free Hemopurification Device |
| 2.4.1.1 | Design and fabricate optimized configuration(s) of hemopurification device(s) that contain(s) a combination of hemofilters, plasma filters, and affinity columns. |
Milestones
| M6: | Define Aethlon’s GMP manufacturing process and revise and upgrade Aethlon’s quality procedures and policies to the current state of the art. |
| 2.4.2 | Subtask 4: Target Capture in Combined Agent Cartridge |
| 2.4.2.1 | Evaluate contribution of manufacturing process variables to binding capacity of affinity resin. |
| 2.4.2.2 | Determine capacity requirements of affinity resin to multiple simultaneous targets. |
| 2.4.2.3 | Perform basic biocompatibility tests for the combination ADAPT device to confirm the combination cartridge does not present additional risk. |
| 2.4.2.4 | Finish construction and delivery of 25 experimental cartridges for testing by the system integrator. |
Milestones
| M7: | Target capture > 90% in 24 hours (12 months) for at least 3 targets ex vivo in blood or blood components using the optimized cartridge. |
| M8: | Pass biocompatibility tests for the combination ADAPT device. |
| M9: | Construct and deliver of 25 prototype cartridges for testing by the system integrator. |
| 2.5 | Option 3 (Year 4) |
| 2.5.1 | Subtask 1a: Anticoagulant-free Hemopurification Device |
| 2.5.1.1 | Complete Aethlon’s GMP procedure and establish and maintain all GMP documentation for the company. |
Milestones
| M11: | Develop a strategic plan for developing an alternate method of producing GNA by cloning the gene into an alternate vector and identify potential partners for such production. |
| 2.5.2 | Subtask 4: Target Capture in Combined Agent Cartridge |
2.5.2.2 Finish construction and delivery of 50 prototype cartridges for testing by the system integrator. The cartridges will need to be made available (packaged, labeled, sterilized and qualified) to the system integrator.
Milestones
M13: Construct and deliver of 50 prototype cartridges for testing by the system integrator.
2.6 Option 4 (Year 5)
2.6.1 Subtask 5: Testing of final product by System Integrator
2.6.1.1 System integrator acceptance of the hemofilter device.
2.6.1.3 Quantify the degree to which the MERS virus can be extracted from circulation in vitro using miniature Hemopurifiers.
2.6.1.4 Prepare and present Final report for DARPA.
Milestones
M12: System Integrator approval of a sepsis precursor ADAPT treatment cartridge
| 5 |
| 3.0 | Program Management and Reviews |
| 3.1 | Program Management Plan |
The contractor shall develop a Program Management Plan. A graphical representation of this plan (Gantt chart is one example) identifying major tasks and their task leaders, milestones of the major task and their completion dates shall be generated. In addition, a graphical representation of budget shall be generated.
| 3.2 | Kick-off Meeting |
The contractor shall participate in a kick-off meeting within 60 days of contract award. The purpose of this meeting is to introduce key program personnel, discuss the proposed tasking, present the program schedule and milestones and the initial Program Management Plan.
| 3.3 | Quarterly Reviews |
The contractor shall hold quarterly reviews for the duration of this effort. The purpose of these reviews is to present a summary of work completed and milestones met, discuss any problems encountered, update the program schedule, present the program financial status, and discuss remaining work.
| 3.4 | Final Contract Review |
A final contract review held in place of the last quarterly review shall be hosted by the principal contractor. The purpose of this review is to present a summary of all work completed and milestones accomplished and to discuss any relevant future efforts similar to the contract that may be pursued.
4.0 Deliverables
The reports and presentation materials are to be delivered in accordance with the attached Data Delivery Matrix.
(End of SOW).
| 6 |
SECTION G - CONTRACT ADMINISTRATION DATA
The following have been modified:
SCHEDULE OF PAYMENT MILESTONES
12 month duration base period (Year 1)
| Milestone | Month | Payable Milestone | GOVT CONTRIBUTION |
| Subtask 1a | Anticoagulant-free Hemopurification Device | ($908,384) | |
| 2.2.1.1 | 1 | Write requirements definition for the extracorporeal blood purification system and acquire necessary equipment. | ($358,284) |
|
2.2.1.2 |
3 |
Fabricate breadboard prototypes for anticoagulation-free anti-sepsis extracorporeal system (ASEPSYS) device. Fabricate prototype blood tubing sets. Acquire anti- thrombogenic surface-modified hollow fiber plasma separators. |
($183,367) |
|
2.2.1.3 |
6 |
Assemble and test breadboard ASEPSYS devices. Evaluate the use of different techniques and approaches to eliminating anticoagulants. |
($183,367) |
|
2.2.1.4 |
12 |
Obtain all necessary IRB documentation and obtain both institutional and Government (SSC-Pacific) approval in accordance with IRB documentation submission guidance prior to conducting human or animal testing. |
($183,367) |
|
Subtask 2 & 4 |
Removal of Sepsis Precursors | ($1,066,663) | |
|
2.2.2.1 |
2 |
Begin to develop the Aethlon’s ADAPT device to efficiently capture sepsis precursors and acquire important equipment and supplies |
($416,424) |
|
2.2.2.2 |
5 |
Perform initial screening of the different proposed capture agents by measuring binding affinity and kinetics using surface plasmon resonance (SPR) or biolayer surface interferometry (BLI). |
($216,747) |
| 2.2.2.3 | 8 | Perform preliminary quantitative real time PCR to measure viral load, and specific DNA or RNA targets. | ($216,747) |
| M2 | 12 | Target capture > 50% in 24 hours for at least 1 target in blood or blood components | ($216,747) |
| Total | ($1,975,047) | ||
| 7 |
12 month duration option #1 period (Year2)
| Milestone | Month | Payable Milestone | GOVT CONTRIBUTION |
| Subtask 1a | Anticoagulant-free Hemopurification Device | ($782,322) | |
|
2.3.2.1 |
15 |
Demonstrate the effectiveness of the prototype device in vivo in animals preventing platelet activation or clotting in at least a 2 hour blood pumping experiment at 75 mL/min blood flow. |
($195,581) |
|
2.3.2.2 |
18 |
Formulate initial design based on work from previous phase. Begin to build and test selected instrument design and tubing sets. |
($195,581) |
|
2.3.2.3 |
21 |
Write and test software. Conduct ergonomic research. Begin discussions with System Integrator. |
($195,581) |
|
M3 |
24 |
Complete fabrication and testing of prototype instrument. Research literature and patent database for technical. |
($195,581) |
| Subtask 2 | Removal of Sepsis Precursors | ($835,124) | |
|
2.3.3.1 |
15 |
Build the ADAPT capture cartridges with the identified affinity agents. Measure the rate of capture of the specific targets from in ex vivo recirculation experiments from cell culture and blood. |
($208,781) |
| M4 | 18 | Target capture > 50% in 24 hours for at least 5 targets in blood or blood components. | ($208,781) |
|
2.3.3.2 |
21 |
Cartridge construction with optimized affinity matrix design for each potential target. Complete the capture agent screening. |
($208,781) |
| M5 | 24 | Target capture > 90% in 24 hours for at least 3 targets in blood or blood components. | ($208,781) |
| Total | ($1,617,446) | ||
| 8 |
12 month duration option #2 period (Year 3)
| Milestone | Month | Payable Milestone | GOVT CONTRIBUTION |
| Subtask 1a | Anticoagulant-free Hemopurification Device | ($372,328) | |
|
2.4.1.1 |
27 |
Design and fabricate optimized configuration(s) of hemopurification device(s) that contain(s) a combination of hemofilters, plasma filters, and affinity columns. |
($186,164) |
|
M6 |
36 |
Define Aethlon’s GMP manufacturing process and revise and upgrade Aethlon’s quality procedures and policies to the current state of the art. |
($187,164) |
| Subtask 2+4 | Target Capture in Combined Agent Cartridge | ($720,726) | |
|
2.4.2.1 |
27 |
Evaluate contribution of manufacturing process variables to binding capacity of affinity resin. |
($197,362) |
|
2.4.2.2 |
33 |
Determine capacity requirements of affinity resin to multiple simultaneous targets. |
($197,362) |
| 2.4.2.3 | 30 | Perform biocompatibility tests for the combination ADAPT device to confirm the combination cartridge does not present additional risk. | ($78,641) |
| 2.4.2.4 | 36 | Finish construction and delivery of 25 experimental cartridges for testing by the system integrator. | ($50,000) |
|
M9 |
36 |
Target capture > 90% in 24 hours (12 months) for at least 3 targets ex vivo in blood or blood components using the optimized cartridge. |
($197,361) |
| Total | ($1,093,054) | ||
12 month duration option #3 period (Year 4)
| Milestone | Month | Payable Milestone | GOVT CONTRIBUTION |
| Subtask 1a | Anticoagulant-free Hemopurification Device | ($276,172) | |
|
2.5.1.1 |
45 |
Complete Aethlon’s GMP procedure and establish and maintain all GMP documentation for the company. | ($90,008) |
|
M11 |
48 |
Develop a strategic plan for developing an alternate method of producing GNA by cloning the gene into an alternate vector and identify potential partners for such production. |
($186,164) |
| Subtask 2+4 | Target Capture in Combined Agent Cartridge | ($296,964) | |
| 2.5.2.2 | 48 | Finish construction and begin delivery of 50 prototype cartridges for testing by the system integrator. | ($296,964) |
| Total | ($759,300) | ||
| 9 |
12 month duration option #4 period (Year 5)
| Milestone | Month | Payable Milestone | GOVT CONTRIBUTION |
| Subtask 5 | Testing of final product by System Integrator | ($581,157) | |
|
2.6.1.1 |
51 |
System integrator acceptance of the hemofilter device. |
($193,719) |
|
2.6.1.3 |
57 |
Quantify the degree to which the MERS virus can be extracted from circulation in vitro using miniature Hemopurifiers. |
($193,719) |
| 2.6.1.4 | 60 | Prepare and present Final Report for DARPA. | ($193,719) |
| Total | ($581,157) | ||
(End of Milestone Schedule)
(End of Summary of Changes)
| 10 |