PRESENTATION
Published on June 7, 2016
Exhibit 99.1

A e thlon Medical, Inc . Nasdaq : AEMD BIO International Convention San Francisco June 7, 2016 Jim Joyce Chairman, CEO
| 1 |

FORWARD LOOKING STATEMENTS The following presentation may contain predictions, estimates, and other forward looking statements that involve risks and uncertainties, including whether and when our products are successfully developed and introduced ; market acceptance of the A e thlon ADAPT™ and ELLSA™ platform technologies, the H e mopurifier ® and other product offerings ; regulatory delays, manufacturing delays, and other risks detailed in our SEC filings, which are accessible at www . sec . gov or on our website : www . A e thlonMedical . com
| 2 |

IMMUNOTHERAPEUTIC TECHNOLOGIES TO COMBAT INFECTIOUS DISEASE & CANCER
| 3 |

SAVE LIVES
| 4 |

Summary • Clinical - stage Company • Lead therapeutic – The Aethlon Hemopurifier ® – B road - spectrum countermeasure against emerging pandemic threats – Discovered to capture cancer - promoting exosomes • Significant unmet need in cancer care • Exosome Sciences, Inc. (diagnostic subsidiary) – ELLSA™ exosome isolation platform (virus & cancer) – Discovered a candidate biomarker to diagnose CTE
| 5 |

The A e thlon Hemopurifier® Broad - s pectrum e limination of circulating viruses, viral particles and bacterial toxins
| 6 |

DEPLOYED WITHIN THE GLOBAL INFRASTRUCTURE OF DIALYSIS & CRRT MACHINES
| 7 |

The Hemopurifier ® Mechanism of Action • Rapid separation of disease targets from the circulatory blood path • Capture of disease targets through adherence to surface structure that provides immune - evasion – Lectin - affinity attachment to high - mannose signature that cloaks pathogen targets from immune surveillance
| 8 |

The Hemopurifier ® establishes a therapeutic strategy to address the hundreds of viruses that are not treatable with antiviral drug agents .
| 9 |

The A e thlon Hemopurifier® FDA approved IDE study currently being conducted
| 10 |

Previous Human Treatment Experiences
| 11 |
BROAD - SPECTRUM HUMAN TREATMENT EXPERIENCES HIV - AIDS Hepatitis - C Ebola
| 12 |

The Treatment of Ebola Virus Frankfurt University Hospital Special approval from The Federal Institute for Drugs and Medical Devices (BfArM)
| 13 |
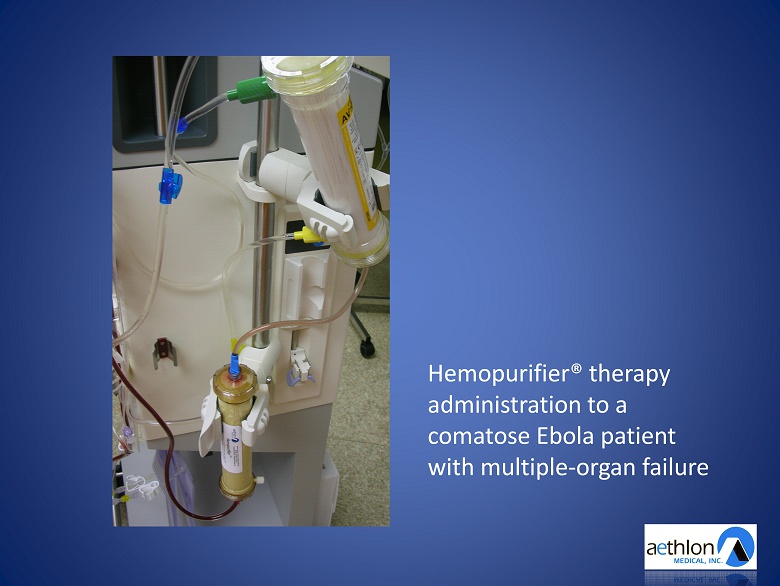
Hemopurifier® therapy administration to a comatose Ebola patient with multiple - organ failure
| 14 |

Dr. Stefan Büttner Holding Hemopurifier® After Ebola Treatment
| 15 |

Ebola Treatment Data Presented By Dr. Helmut Geiger American Society of Nephrology Annual Meeting • 6.5 hour Hemopurifier® therapy administration • Pre - treatment viral load: 400,000 copies/ml • Post - treatment viral load: 1,000 copies/ml • Patient recovered and returned home
| 16 |

Extracorporeal Virus Elimination for the Treatment of Severe Ebola Virus Disease – First Experience with Lectin Affinity Plasmapheresis • Stefan Bü ttner a Benjamin Koch a Olga Dolnik b Markus Eickmann b Tilo Freiwald a Sarah Rudolf a Jü rgen Engel a Stephan Becker b Claudio Ronco c Helmut Geiger a • a Medical Clinic III, Department of Nephrology, University Hospital Frankfurt, Frankfurt am Main, and b Institute of Virology, Philipps University Marburg, Marburg, Germany; c Department of Nephrology, Dialysis and Transplantation, International Renal Research Institute of Vicenza (IRRIV), San Bortolo Hospital, Vicenza, Italy • Published: Journal of Blood Purification
| 17 |

“Top 25 Best Inventions” “11 Most Remarkable Advances in Healthcare”
| 18 |

Pre - clinical Validations ( Reported t o date )
| 19 |

In Vitro Hemopurifier ® Validations • Viruses – Hepatitis C, HIV, Ebola, H1N1 Swine Flu, H5N1 Bird Flu, 1918 Spanish Flu (reconstructed), Monkey Pox, West Nile Virus, Chikungunya and Dengue • Viral Toxins • Ebola glycoprotein ( gp ), HIV gp120, HIV NEF - exosomes • Bacterial Toxins • L ipopolysaccharide (LPS) and L ipoteichoic acid (LTA) • Tumor - derived E xosomes
| 20 |

Initiated tumor - derived exosome research in 2006 Considered c ellular debris w ith n o b iological f unction CANCER
| 21 |

2016 Tumor - derived exosomes are significant therapeutic and diagnostic targets
| 22 |
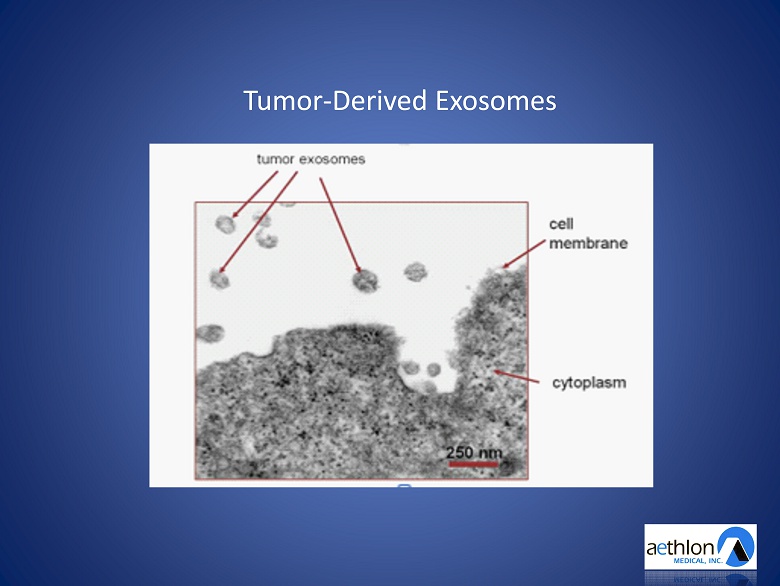
Tumor - Derived Exosomes
| 23 |

Tumor - Derived Exosomes A Significant Unmet Medical Need in Cancer • The seeds of cancer metastasis – Metastasis contributes to 90% of cancer deaths – The mystery of the Soil & Seed Theory of 1889 • Tumor - derived exosomes promote: – Tumorigenesis – Cancer progression – Angiogenesis – Immune - evasion – Resistance to radiation and chemotherapeutic drugs
| 24 |

The Hemopurifier ® provides an adjunct strategy to optimize emerging immuno - oncology drugs or improve the benefit of chemotherapeutic regimens
| 25 |

| 26 |

ELLSA™ (Enzyme - linked lectin - specific assay) Proprietary exosome isolation platform
| 27 |

ELLSA™ • Isolates disease - origin exosomes from bodily fluids – ELLSA step + specific antibody step to diagnose and monitor a variety of disease indications – Urine based proof - of - principal established in HIV • Identified HIV - specific exosomes in 111 HIV - infected individuals, but not in the urine of 35 HIV negative control subjects. • A potential noninvasive global strategy to diagnose HIV infection. • In collaboration with Morehouse School of Medicine, who conducted the study
| 28 |
Chronic Traumatic Encephalopathy (CTE)
| 29 |

• Discovery of CTE biomarker candidate ( TauSome ™) • TauSome testing was conducted in the DETECT study – In collaboration with the Boston University CTE Center • Principal Investigator: Robert Stern Ph.D. – Goal: a test that could detect CTE in living individuals – 78 former NFL players (high risk CTE group) – 16 athlete controls (low risk CTE group)
| 30 |

Preliminary Study of Plasma Exosomal Tau as a Potential Biomarker for Chronic Traumatic Encephalopathy Stern , Tripodis , Baugh, Fritts , Martin, Chaisson , Cantu, Joyce, Shah, Ikezu , Zhang, Gercel - Taylor, & Taylor J Alzheimer’s Disease, 2016 • Findings suggest that TauSome plasma levels may be an accurate, noninvasive CTE biomarker – TauSome levels significantly higher in the NFL group – TauSome levels correlated with cognitive decline
| 31 |
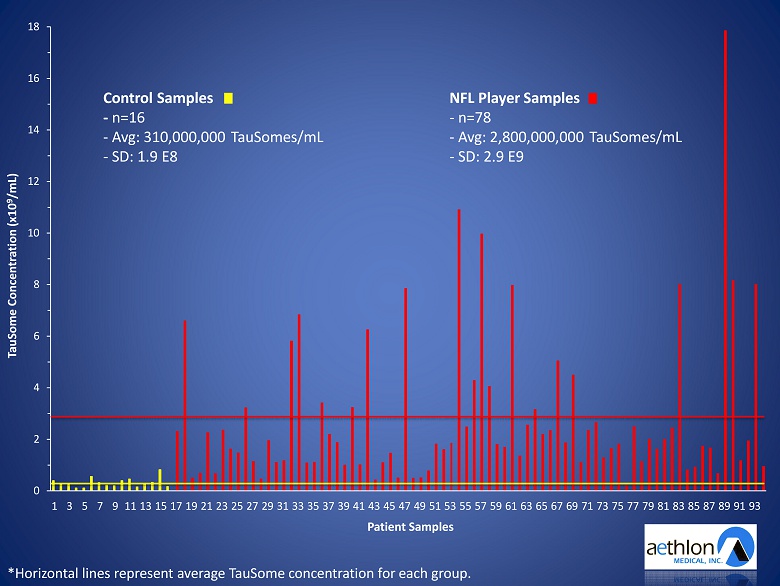
0 2 4 6 8 10 12 14 16 18 1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39 41 43 45 47 49 51 53 55 57 59 61 63 65 67 69 71 73 75 77 79 81 83 85 87 89 91 93 TauSome Concentration (x10 9 /mL) Patient Samples *Horizontal lines represent average TauSome concentration for each group. Control Samples - n=16 - Avg : 310,000,000 TauSomes /mL - SD: 1.9 E8 NFL Player Samples - n=78 - Avg : 2,800,000,000 TauSomes /mL - SD: 2.9 E9
| 32 |

• TauSome Next Steps (more testing required) – Validate that TauSomes are brain - derived – Refine technique to ELLSA - antibody or other scalable methodology – Initiate TauSome studies in other tauopathies – Initiate TauSome studies in other high - risk CTE groups – TauSome testing to be included in new (up to a 17 - sites) CTE study funded by a $ 16 million NIH grant
| 33 |

Summary • Clinical - stage Company • Lead therapeutic – The Aethlon Hemopurifier ® – B road - spectrum countermeasure against emerging pandemic threats – Discovered to capture cancer - promoting exosomes • Significant unmet need in cancer care • Exosome Sciences, Inc. (diagnostic subsidiary) – ELLSA™ exosome isolation platform (virus & cancer) – Discovered a candidate biomarker to diagnose CTE
| 34 |

IMMUNOTHERAPEUTIC TECHNOLOGIES TO COMBAT INFECTIOUS DISEASE & CANCER
| 35 |

SAVE LIVES
| 36 |

Acknowledgement and Thanks • Team Aethlon • Battelle Memorial Research Institute • Boston University CTE Center • DaVita Med Center Dialysis • Defense Advanced Research Projects Agency (DARPA ) • Frankfurt University Hospital • Medanta Medicity , Fortis and Apollo Hospitals • Morehouse School of Medicine • National Institute of Virology (NIV) Pune, India • Philipps University Marburg • U.S. Army Medical Research Institute for Infectious Diseases (USAMRIID ) • U.S. Centers for Disease Control (CDC)
| 37 |

CONTACT: A e thlon Medical, Inc 9635 Granite Ridge Drive Suite 100 San Diego, California 92123 Jim Joyce Chairman & CEO jj@aethlonmedical.com 858.459.7800 x301
| 38 |