POWERPOINT PRESENTATION
Published on June 3, 2016
Exhibit 99.1

A e thlon Medical, Inc . Nasdaq : AEMD SACHS IMMUNO - ONCOLOGY BD&L INVESTMENT FORUM JUNE 3, 2016 Jim Joyce Chairman, CEO
| 1 |

FORWARD LOOKING STATEMENTS The following presentation may contain predictions, estimates, and other forward looking statements that involve risks and uncertainties, including whether and when our products are successfully developed and introduced ; market acceptance of the A e thlon ADAPT™ and ELLSA™ platform technologies, the H e mopurifier ® and other product offerings ; regulatory delays, manufacturing delays, and other risks detailed in our SEC filings, which are accessible at www . sec . gov or on our website : www . A e thlonMedical . com
| 2 |

IMMUNOTHERAPEUTIC TECHNOLOGIES TO COMBAT INFECTIOUS DISEASE & CANCER
| 3 |

TUMOR - DERIVED EXOSOMES A Basis for Therapeutic and Diagnostic Partnering
| 4 |

Initiated tumor - derived exosome research in 2006 Considered c ellular debris w ith n o b iological f unction
| 5 |

2016 Tumor - derived exosomes are significant therapeutic and diagnostic targets
| 6 |
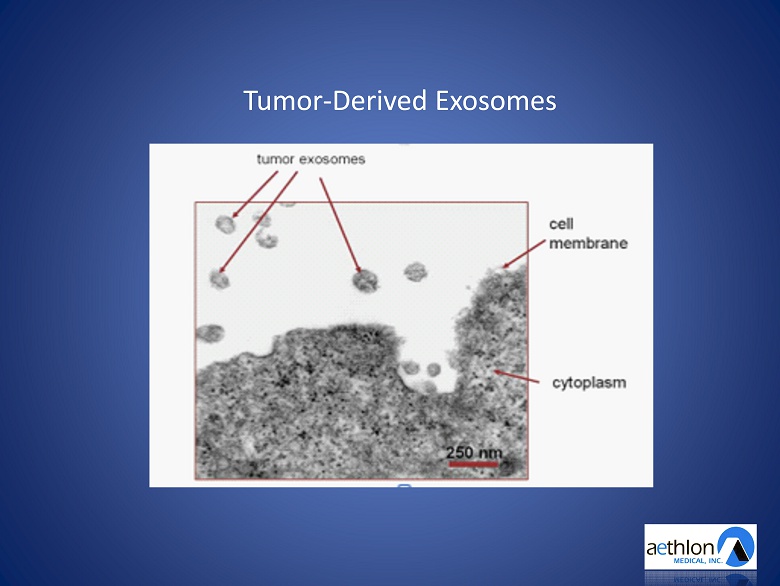
Tumor - Derived Exosomes
| 7 |
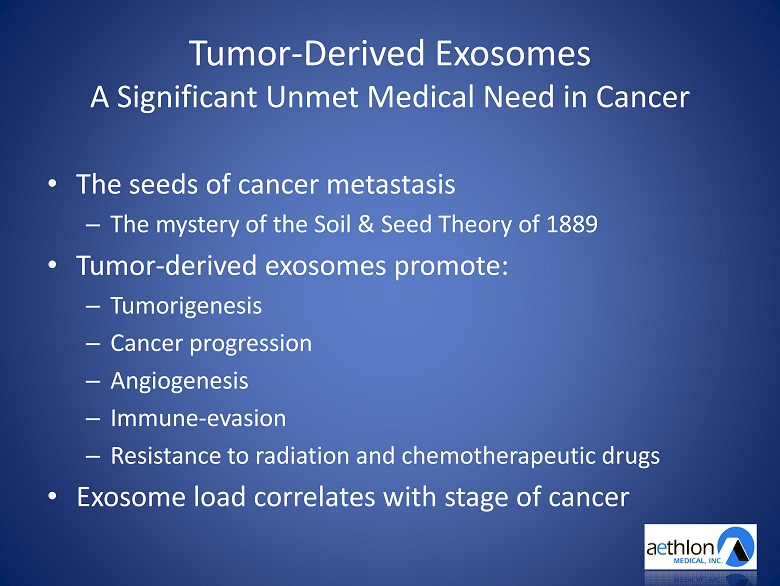
Tumor - Derived Exosomes A Significant Unmet Medical Need in Cancer • The seeds of cancer metastasis – The mystery of the Soil & Seed Theory of 1889 • Tumor - derived exosomes promote: – Tumorigenesis – Cancer progression – Angiogenesis – Immune - evasion – Resistance to radiation and chemotherapeutic drugs • Exosome load correlates with stage of cancer
| 8 |

The A e thlon Hemopurifier® Broad - s pectrum e limination of circulating viruses, bacterial toxins and tumor - derived exosomes
| 9 |

DEPLOYED WITHIN THE GLOBAL INFRASTRUCTURE OF DIALYSIS & CRRT MACHINES
| 10 |

The A e thlon Hemopurifier® FDA approved IDE study currently being conducted
| 11 |
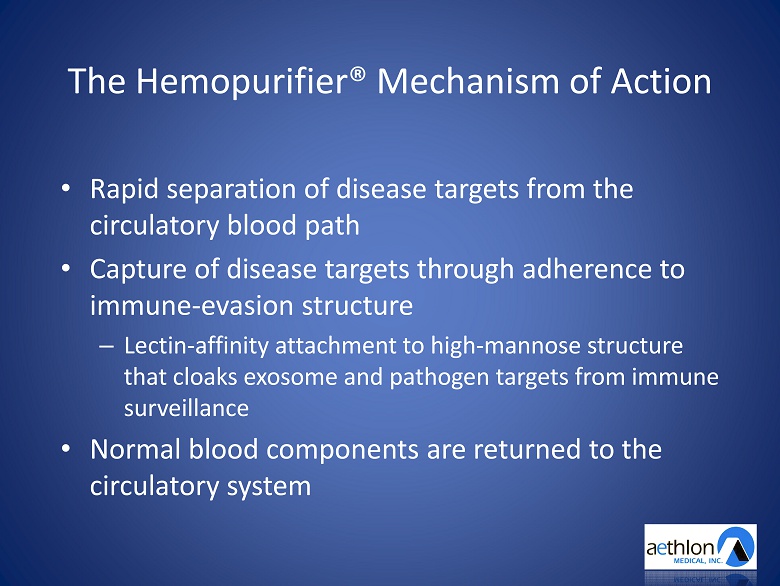
The Hemopurifier ® Mechanism of Action • Rapid separation of disease targets from the circulatory blood path • Capture of disease targets through adherence to immune - evasion structure – Lectin - affinity attachment to high - mannose structure that cloaks exosome and pathogen targets from immune surveillance • Normal blood components are returned to the circulatory system
| 12 |

Previous Human Treatment Experiences
| 13 |
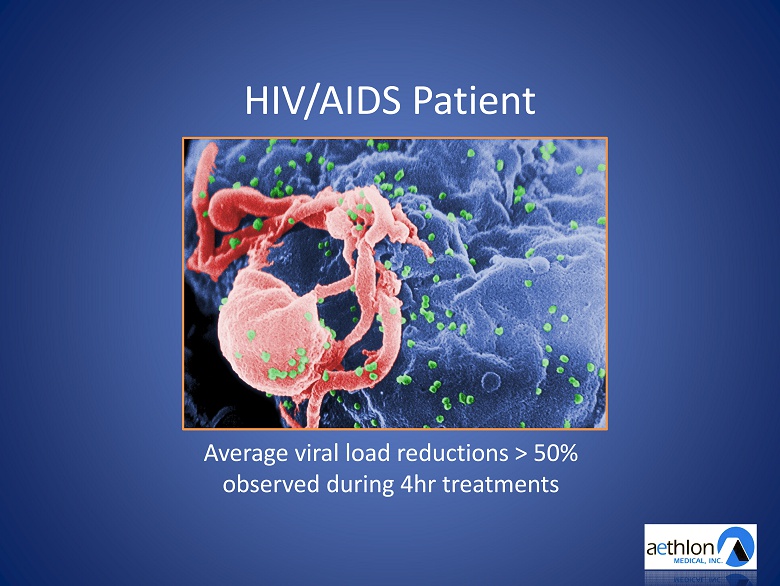
HIV/AIDS Patient Average viral load reductions > 50% observed during 4hr treatments
| 14 |
Hepatitis C Virus (HCV)
| 15 |

HCV Treatment Summary • Demonstrated successful use in interferon - based cure strategy • Up to 300 billion HCV copies captured during single six - hour treatments
| 16 |
Ebola Virus
| 17 |
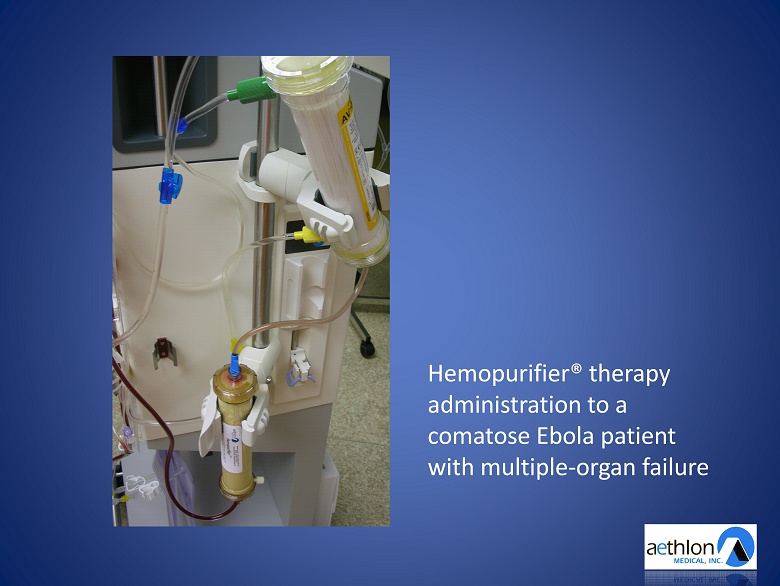
Hemopurifier® therapy administration to a comatose Ebola patient with multiple - organ failure
| 18 |

Dr. Stefan Büttner Holding Hemopurifier® After Ebola Treatment
| 19 |

Ebola Treatment Data Presented By Dr. Helmut Geiger American Society of Nephrology Annual Meeting • 6.5 hour Hemopurifier® therapy administration • Pre - treatment viral load: 400,000 copies/ml • Post - treatment viral load: 1,000 copies/ml • 242 million of Ebola viruses captured • Patient recovered and returned home
| 20 |

“Top 25 Best Inventions” “11 Most Remarkable Advances in Healthcare”
| 21 |
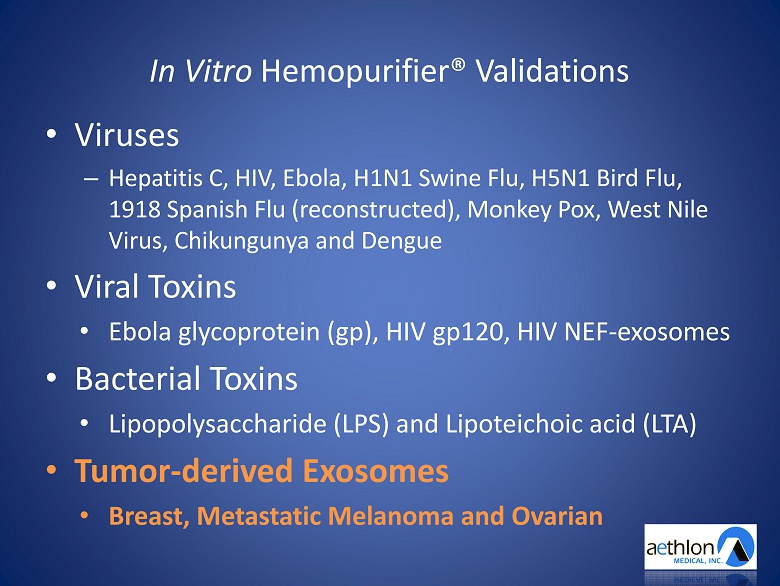
In Vitro Hemopurifier ® Validations • Viruses – Hepatitis C, HIV, Ebola, H1N1 Swine Flu, H5N1 Bird Flu, 1918 Spanish Flu (reconstructed), Monkey Pox, West Nile Virus, Chikungunya and Dengue • Viral Toxins • Ebola glycoprotein ( gp ), HIV gp120, HIV NEF - exosomes • Bacterial Toxins • L ipopolysaccharide (LPS) and L ipoteichoic acid (LTA) • Tumor - derived E xosomes • Breast, Metastatic Melanoma and Ovarian
| 22 |

Expansive Exosome IP Portfolio Issued & Pending Patents Teach • Therapeutic elimination of exosomes • Isolation of exosomes from bodily fluids – p latform to diagnose and monitor cancer • Exosome harvesting – exosomal delivery of biologic & chemotherapeutic agents
| 23 |

Partnering/Collaboration Initiatives
| 24 |
Partnering/Collaboration Initiatives • Therapeutic elimination of exosomes – Preclinical Hemopurifier - d rug study validations • Confirm combination Hemopurifier - drug synergies • Demonstrate non - affinity binding of drug agent • Expand potential therapeutic indications – Clinical program initiatives • Full - circle immuno - oncology treatment regimens • Chemotherapy optimization regimens
| 25 |

Partnering/Collaboration Initiatives • Therapeutic elimination of exosomes • Isolation of exosomes from bodily fluids – p latform to diagnose and monitor cancer • Exosome harvesting – Exosomal delivery of biologic & chemotherapeutic agents
| 26 |

ELLSA™ (Enzyme - linked lectin - specific assay) Proprietary broad - spectrum exosome isolation platform
| 27 |
Partnering/Collaboration Initiatives • Isolation of exosomes from bodily fluids – ELLSA co - development partnerships • ELLSA isolation + antibody step to diagnose and monitor specific disease indications • Urine based proof - of - principal established in HIV
| 28 |
Partnering/Collaboration Initiatives • Therapeutic elimination of exosomes • Isolation of exosomes from bodily fluids – p latform to diagnose and monitor cancer • Exosome harvesting – Exosomal delivery of biologic & chemotherapeutic agents – Contact management to initiate discussions
| 29 |

IMMUNOTHERAPEUTIC TECHNOLOGIES TO COMBAT INFECTIOUS DISEASE & CANCER
| 30 |
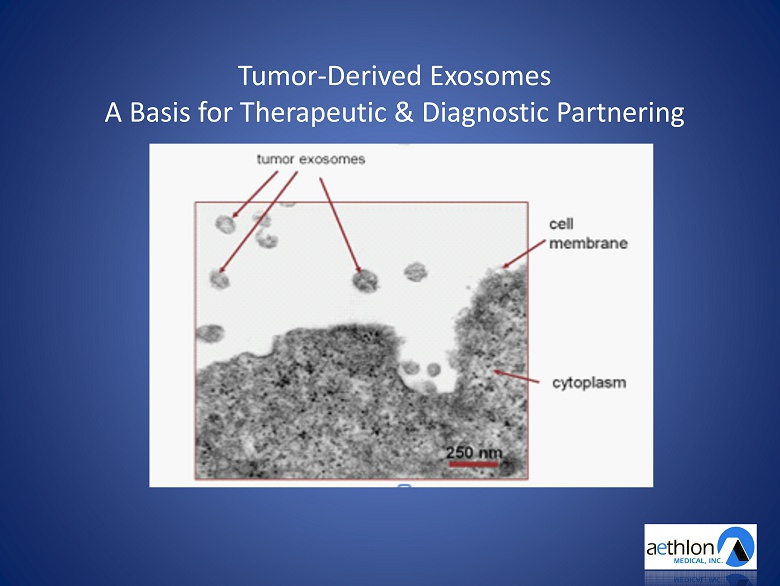
Tumor - Derived Exosomes A Basis for Therapeutic & Diagnostic Partnering
| 31 |

CONTACT: A e thlon Medical, Inc 9635 Granite Ridge Drive Suite 100 San Diego, California 92123 Jim Joyce Chairman & CEO jj@aethlonmedical.com 858.459.7800 x301
| 32 |