PRESENTATION
Published on November 12, 2015
Exhibit 99.1

A e thlon Medical, Inc . Therapeutic Partnering Opportunities IN3 Medical Device Summit November 11, 2015 Jim Joyce Chairman, CEO
| 1 |

FORWARD LOOKING STATEMENTS The following presentation may contain predictions, estimates, and other forward looking statements that involve risks and uncertainties, including whether and when our products are successfully developed and introduced ; market acceptance of the A e thlon ADAPT™ system, the H e mopurifier ® and other product offerings ; regulatory delays, manufacturing delays, and other risks detailed in our SEC filings, which are accessible at www . sec . gov or on our website : www . A e thlonMedical . com
| 2 |

About A e thlon Medical • Nasdaq : AEMD • Headquartered in San Diego, California • We Create Affinity Biofiltration Devices to Treat Life - Threatening Diseases
| 3 |

A e thlon Affinity Biofiltration Technology • Expansive Therapeutic Device Platform – The A e thlon ADAPT™ System • A daptive D ialysis - Like A ffinity P latform T echnology • Intersection of a ffinity c ompounds & advanced p lasma m embrane t echnologies • Rapid elimination of circulating disease targets – Lead product provides clinical pathway into infectious disease and oncology • Being advanced under an FDA approved clinical study
| 4 |
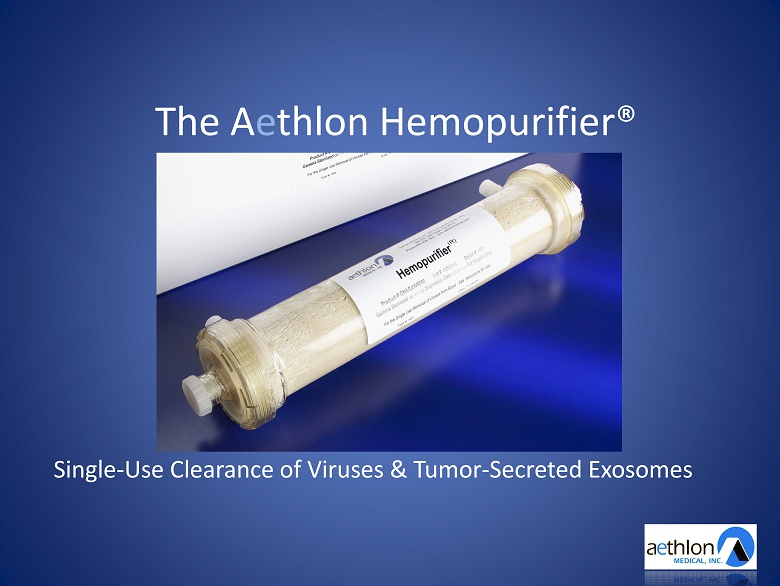
The A e thlon Hemopurifier® Single - Use Clearance of Viruses & Tumor - Secreted Exosomes
| 5 |

DEPLOYED WITHIN THE GLOBAL INFRASTRUCTURE OF DIALYSIS & CRRT MACHINES
| 6 |
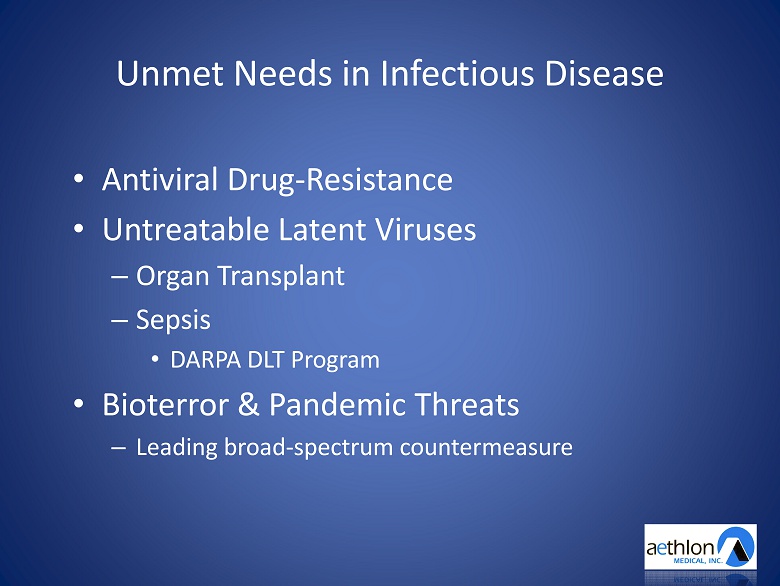
Unmet Needs in Infectious Disease • Antiviral Drug - R esistance • Untreatable Latent Viruses – Organ Transplant – Sepsis • DARPA DLT Program • Bioterror & Pandemic Threats – Leading broad - spectrum countermeasure
| 7 |

| 8 |
| 9 |
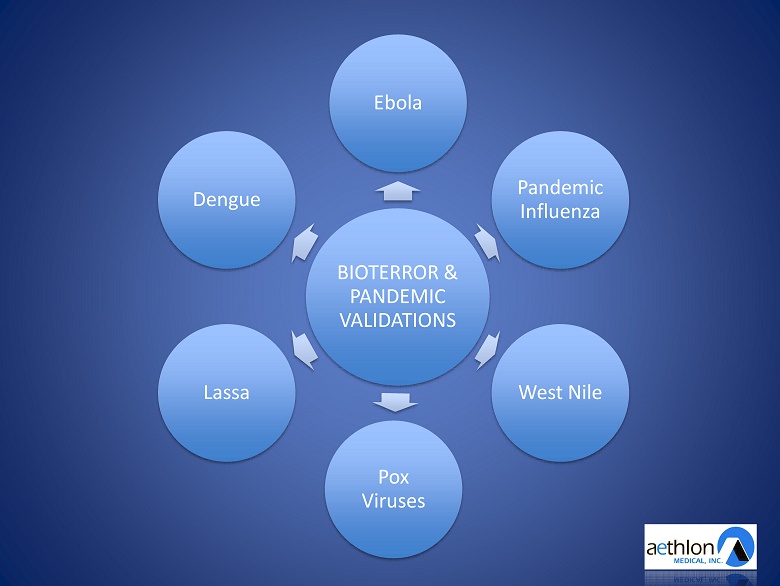
BIOTERROR & PANDEMIC VALIDATIONS Ebola Pandemic Influenza West Nile Pox Viruses Lassa Dengue
| 10 |
Human Treatment Experience HIV, Hepatitis - C and Ebola Virus
| 11 |

A Case Study of Hemopurifier ® Therapy Against an Untreatable Viral Pathogen
| 12 |

The Treatment of Ebola Virus Frankfurt University Hospital Special approval from The Federal Institute for Drugs and Medical Devices (BfArM)
| 13 |
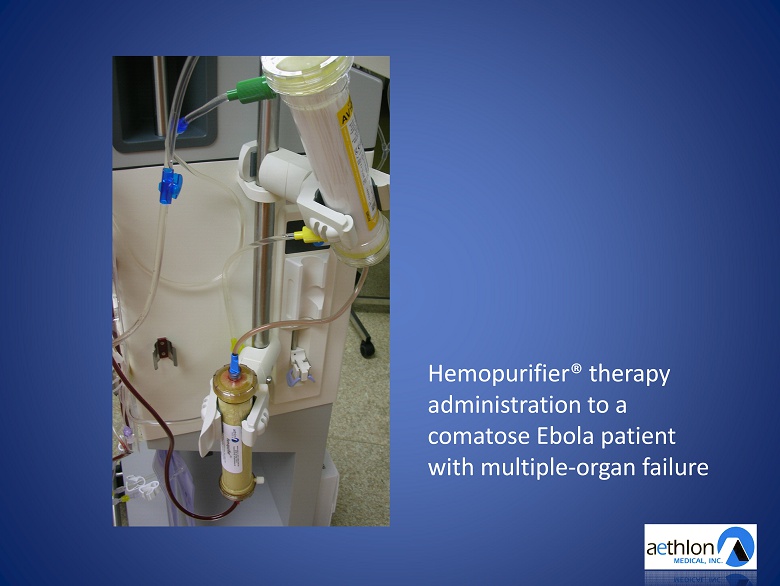
Hemopurifier® therapy administration to a comatose Ebola patient with multiple - organ failure
| 14 |

Dr. Stefan Büttner Holding Hemopurifier® After Ebola Treatment
| 15 |

Ebola Treatment Data Presented By Dr. Helmut Geiger American Society of Nephrology Annual Meeting • 6.5 hour Hemopurifier® therapy administration • Pre - treatment viral load: 400,000 copies/ml • Post - treatment viral load: 1,000 copies/ml • 242 million of Ebola viruses captured • Patient recovered and returned home
| 16 |

Application of the Hemopurifier ® in Cancer SAVE LIVES
| 17 |
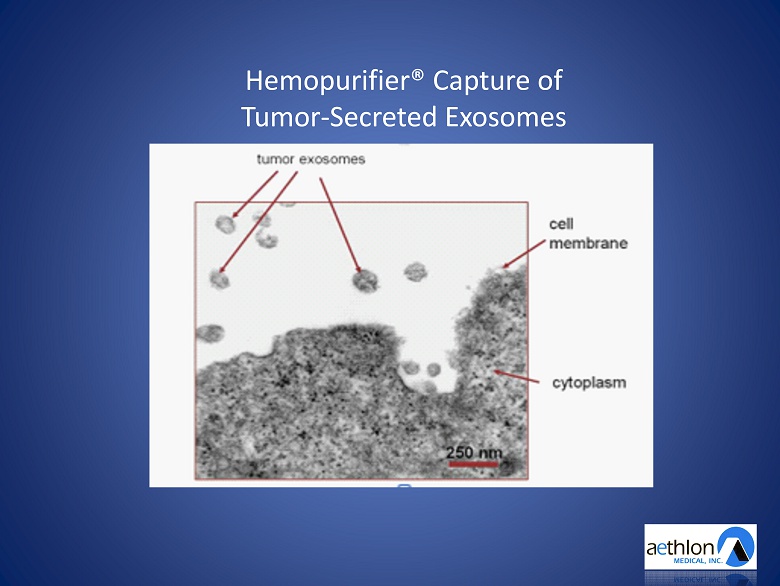
Hemopurifier ® Capture of Tumor - Secreted Exosomes
| 18 |

Tumor - Secreted Exosomes A Significant Unmet Medical Need in Cancer • Trigger apoptosis of immune c ells • Contribute to drug and chemotherapy resistance • Promote angiogenesis • Seed the creation and spread of metastasis • Exosome load correlates with stage of cancer
| 19 |

AS WE CLINICALLY PROGRESS OUR HEMOPURIFIER®, WE WILL PARTNER TO ESTABLISH A PIPELINE OF ADAPT™ BASED THERAPIES
| 20 |

Disease Partnering Characteristics • Must be a life - threatening disease • Limited or no proven therapeutic options • Indications affecting < 4000 per year may be given humanitarian/orphan consideration • Disease promoting factors must be accessible in the circulatory system – Single or multi - target (cocktail) mechanism • Example: R emoval of tumor - secreted exosomes + other oncology targets
| 21 |
Partnering Candidate Profile • Industry, VC and Institutional Healthcare Investors • Academic research teams • Therapeutic organizations interested in testing a device pathway for their affinity compounds
| 22 |

CONTACT: A e thlon Medical, Inc 9635 Granite Ridge Drive Suite 100 San Diego, California 92123 Jim Joyce Chairman & CEO jj@aethlonmedical.com 858.459.7800 x301
| 23 |