POWER POINT PRESENTATION
Published on September 10, 2015
Exhibit 99.1

FORWARD LOOKING STATEMENTS The following presentation may contain predictions, estimates, and other forward looking statements that involve risks and uncertainties, including whether and when our products are successfully developed and introduced ; market acceptance of the A e thlon ADAPT™ system, the H e mopurifier ® and other product offerings ; regulatory delays, manufacturing delays, and other risks detailed in our SEC filings, which are accessible at www . sec . gov or on our website : www . A e thlonMedical . com
| 1 |

A e thlon Medical, Inc . Rodman & Renshaw Conference September 10, 2015 Jim Joyce Chairman, CEO
| 2 |

AFFINITY BIOFILTRATION DEVICES TO TREAT LIFE - THREATENING DISEASES
| 3 |

SAVE LIVES
| 4 |

A e thlon Details • Clinical - stage Therapeutic Organization • Headquartered in San Diego, California • Nasdaq Capital Market: “AEMD” • Market Value: ~ $60 million
| 5 |

A e thlon Technology Highlights • Expansive Therapeutic Device Platform – Lead product provides clinical pathway into infectious disease and cancer – Advancing a sepsis treatment device through contracts with DARPA
| 6 |
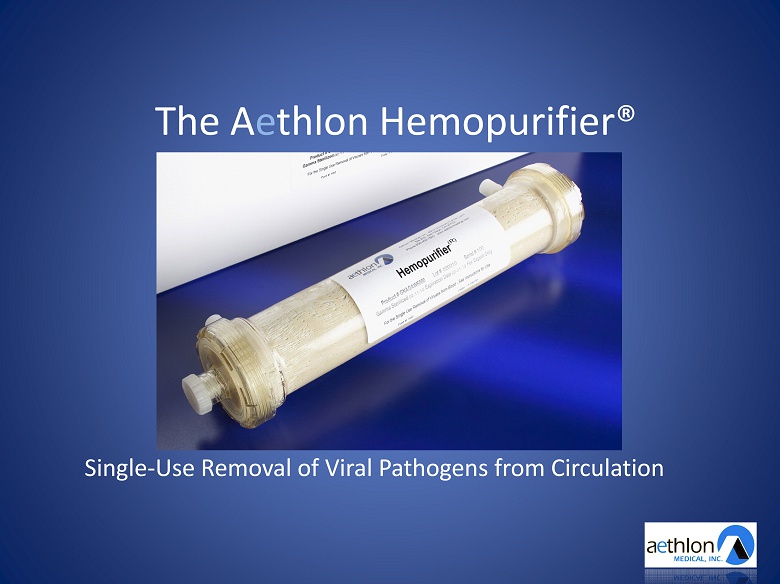
The A e thlon Hemopurifier® Single - Use Removal of Viral Pathogens from Circulation
| 7 |

| 8 |

| 9 |
Rethinking the Treatment of Viral Pathogens • Rapid virus elimination – Prior to cell and organ infection – Elution assay q uantifies virus capture • Targets viral structure vs. inhibiting replication – Allows broad - spectrum virus capture • Addresses shed viral proteins – To augment/preserve host immune response
| 10 |

DEPLOYED WITHIN THE GLOBAL INFRASTRUCTURE OF DIALYSIS & CRRT MACHINES
| 11 |

Can the Hemopurifier® Improve the Benefit of Drug Treatment Regimens?
| 12 |
Hepatitis C Virus (HCV ) Safety & Efficacy Observations Conducted at the Medanta - Medicity Institute
| 13 |

HCV Study Highlights • Safely Administered in Combination with Interferon - Ribavirin Drug Regimen • Improved Rapid Viral Response (RVR) – 58% RVR vs. 10.35% • Based on IDEAL Study of 3070 HCV patients • Two Patients Undetectable at Day Seven • 300 Billion Copies of HCV Captured
| 14 |

Can the Hemopurifier® Address Viral Pathogens Not Treatable with Drug Therapies?
| 15 |

The Treatment of Ebola Virus Frankfurt University Hospital Special approval from The Federal Institute for Drugs and Medical Devices (BfArM)
| 16 |
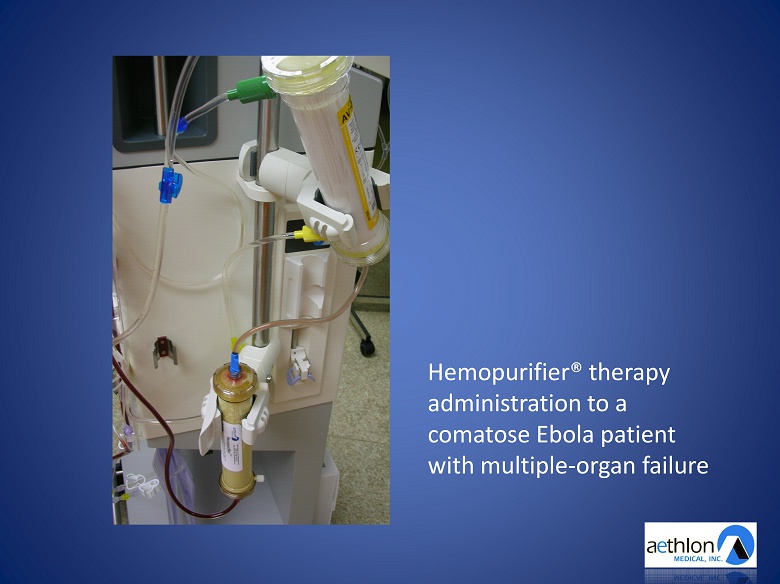
Hemopurifier® therapy administration to a comatose Ebola patient with multiple - organ failure
| 17 |

Dr. Stefan Büttner Holding Hemopurifier® After Ebola Treatment
| 18 |

Ebola Treatment Data Presented By Dr. Helmut Geiger American Society of Nephrology Annual Meeting • 6.5 hour Hemopurifier® therapy administration • Pre - treatment viral load: 400,000 copies/ml • Post - treatment viral load: 1,000 copies/ml • 242 million of Ebola viruses captured • Patient recovered and returned home
| 19 |

Extracorporeal Virus Elimination for the Treatment of Severe Ebola Virus Disease – First Experience with Lectin Affinity Plasmapheresis • Stefan Bü ttner a Benjamin Koch a Olga Dolnik b Markus Eickmann b Tilo Freiwald a Sarah Rudolf a Jü rgen Engel a Stephan Becker b Claudio Ronco c Helmut Geiger a • a Medical Clinic III, Department of Nephrology, University Hospital Frankfurt, Frankfurt am Main, and b Institute of Virology, Philipps University Marburg, Marburg, Germany; c Department of Nephrology, Dialysis and Transplantation, International Renal Research Institute of Vicenza (IRRIV), San Bortolo Hospital, Vicenza, Italy • Published: February 11 , 2015
| 20 |

The A e thlon Hemopurifier® Our Infectious Disease Opportunity
| 21 |

Unmet Needs in Infectious Disease • Antiviral Drug - R esistance – HIV & HCV • Latent Virus Activation – Immune Suppression • Organ Transplants, Sepsis • Bioterror & Pandemic Threats – Leading broad - spectrum countermeasure against known and unknown viruses
| 22 |

BIOTERROR & PANDEMIC VALIDATIONS Ebola Pandemic Influenza West Nile Pox Viruses Lassa Dengue
| 23 |

Currently Approved Human Treatment Protocols • U.S. Food and Drug Administration (FDA) – IDE Feasibility Study (10 Patient) • HCV Infected ESRD Patients (Study Initiated) • Single - site at DaVita Med Center Dialysis in Houston • Gateway to Pivotal Studies – Ebola Treatment Protocol • Health Canada – Ebola Treatment Protocol
| 24 |

ONE MORE THING
| 25 |

The A e thlon Hemopurifier®
| 26 |

CANCER SAVE LIVES
| 27 |
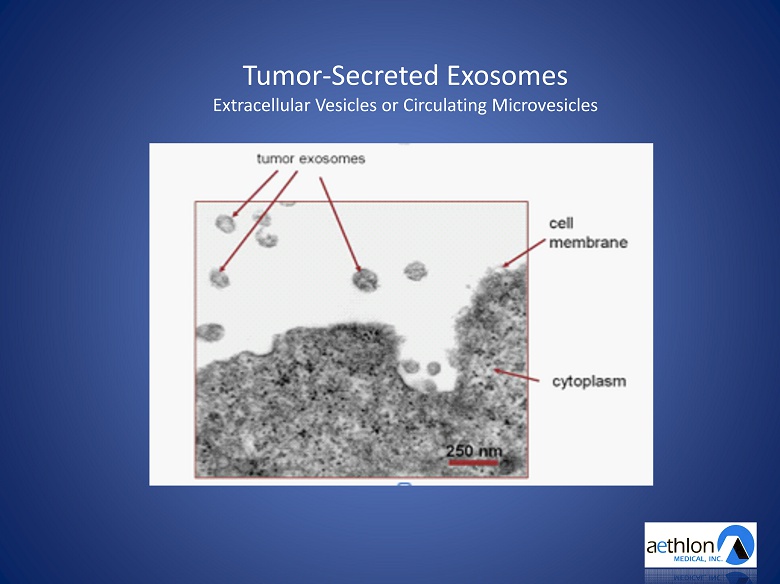
Tumor - Secreted Exosomes Extracellular Vesicles or Circulating Microvesicles
| 28 |

May, 2014 Trends in Molecular Medicine "Extracellular Vesicles: Emerging Targets for Cancer Therapy"
| 29 |

Tumor - Secreted Exosomes A Significant Unmet Medical Need in Cancer • Trigger apoptosis of immune c ells • Contribute to drug and chemotherapy resistance • Promote angiogenesis • Seed the creation and spread of metastasis • Exosome load correlates with stage of cancer
| 30 |

Clinical Progression Objectives 6 - 12 Months • Complete FDA Approved IDE Study – Prepare pivotal study submission • Advance HUD submissions – Disease conditions affecting < 4000 per yr. in the U.S. • Advance Cancer Treatment Programs
| 31 |

Near - Term Corporate Objectives Outlined During Quarterly Call on 8/13/15 • Complete Principal Investigator Transition – IDE Study in Houston • Secure Year - 5 DARPA Contract • Initiate New Clinical Collaborations • Initiate Cancer Study at UC Irvine Medical Center • Submit NFL Detect Study (CTE Tausome ® Data) Manuscript for Publication ( Exosome Sciences)
| 32 |

Near - Term Corporate Objectives Outlined During Quarterly Call on 8/13/15 PROGRESS SINCE • Secure Year - 5 DARPA Contract – Received Verbal Confirmation of Contract Renewal • Initiate New Clinical Collaborations – Initiated Chikungunya Virus Capture Study • Initiate Cancer Study at UC Irvine Medical Center – First Patient Enrolled (Ovarian Cancer) • Submit NFL Detect Study (CTE Tausome ® Data) Manuscript for Publication ( Exosome Sciences) – Submitted
| 33 |

AFFINITY BIOFILTRATION DEVICES TO TREAT LIFE - THREATENING DISEASES
| 34 |

OUR LEAD PRODUCT PROVIDES A CLINICAL PATHWAY TO ADDRESS INFECTIOUS DISEASE & CANCER
| 35 |

SAVE LIVES
| 36 |