PRESENTATION
Published on June 18, 2015
Exhibit 99.1

FORWARD LOOKING STATEMENTS The following presentation may contain predictions, estimates, and other forward looking statements that involve risks and uncertainties, including whether and when our products are successfully developed and introduced ; market acceptance of the A e thlon ADAPT™ system, the H e mopurifier ® and other product offerings ; regulatory delays, manufacturing delays, and other risks detailed in our SEC filings, which are accessible at www . sec . gov or on our website : www . A e thlonMedical . com

A e thlon Medical, Inc . BIO INTERNATIONAL CONVENTION JUNE 18 TH , 2015 JIM JOYCE CHAIRMAN, CEO

THERAPEUTIC BIOFILTRATION DEVICES TO ADDRESS LIFE - THREATENING DISEASES

SAVE LIVES

A e thlon Highlights • Expansive Therapeutic Platform – Lead product provides clinical pathway to address cancer and infectious viral pathogens • DOD Government Contractor (DARPA) – Dialysis - like therapeutics program to treat sepsis
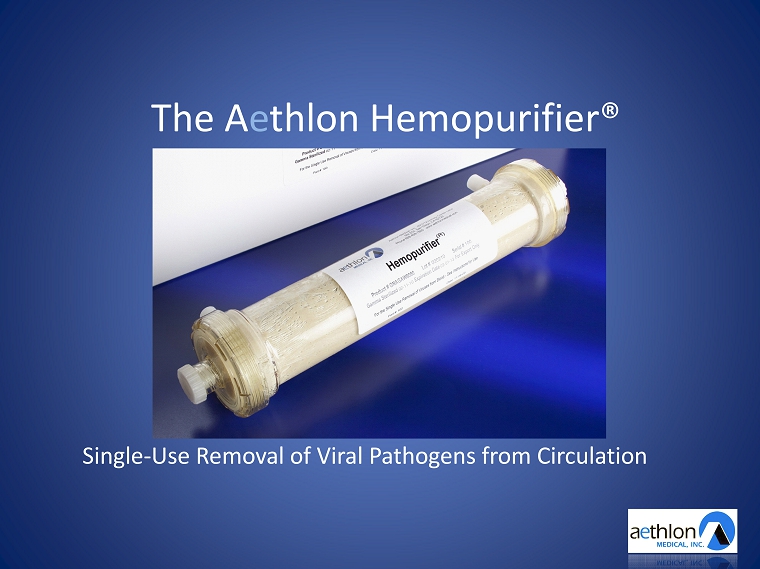
The A e thlon Hemopurifier® Single - Use Removal of Viral Pathogens from Circulation
BROAD - SPECTRUM TREATMENT EXPERIENCE HIV - AIDS Hepatitis - C Ebola

Top 25 Best Inventions of 2014
The 11 Most Remarkable Advances in Healthcare in 2014
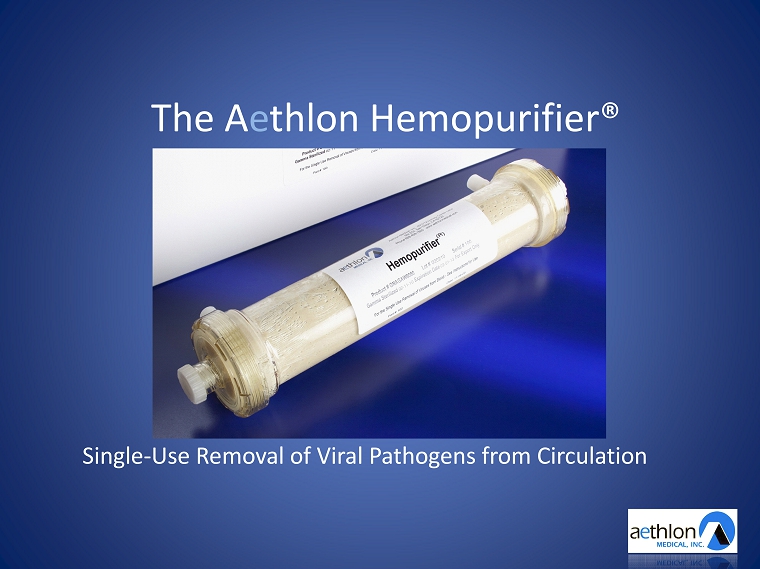
The A e thlon Hemopurifier® Single - Use Removal of Viral Pathogens from Circulation
Rethinking the Treatment of Viral Pathogens • Rapid virus elimination – Prior to cell and organ infection • Targets viral structure vs. inhibiting replication – Allows broad - spectrum virus capture • Addresses virally shed glycoproteins – To augment and preserve host immune response

DEPLOYED WITHIN THE GLOBAL INFRASTRUCTURE OF DIALYSIS & CRRT MACHINES
Recent Clinical Advancements • Disclosure of HCV treatment results • Reported the treatment of Ebola patient • First clinical protocols approved by FDA – Initiated IDE Feasibility Study in HCV Patients – Ebola Treatment Protocol • Health Canada clinical approval (Ebola)

Ebola Virus as a Therapeutic Model

Reported Ebola Treatment Results Frankfurt University Hospital Special approval from The Federal Institute for Drugs and Medical Devices (BfArM)
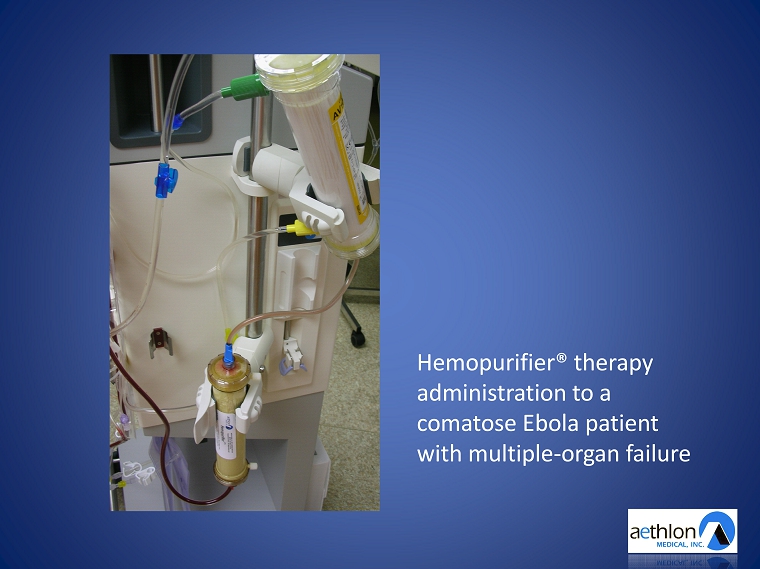
Hemopurifier ® therapy administration to a comatose Ebola patient with multiple - organ failure

Ebola Treatment Data Presented By Dr. Helmut Geiger American Society of Nephrology Annual Meeting • 6.5 hour Hemopurifier® therapy administration • Pre - treatment viral load: 400,000 copies/ml • Post - treatment viral load: 1,000 copies/ml • 242 million of Ebola viruses captured • Patient recovered and returned home

Dr. Stefan Büttner Holding Hemopurifier® After Ebola Treatment

Extracorporeal Virus Elimination for the Treatment of Severe Ebola Virus Disease – First Experience with Lectin Affinity Plasmapheresis • Stefan Bü ttner a Benjamin Koch a Olga Dolnik b Markus Eickmann b Tilo Freiwald a Sarah Rudolf a Jü rgen Engel a Stephan Becker b Claudio Ronco c Helmut Geiger a • a Medical Clinic III, Department of Nephrology, University Hospital Frankfurt, Frankfurt am Main, and b Institute of Virology, Philipps University Marburg, Marburg, Germany; c Department of Nephrology, Dialysis and Transplantation, International Renal Research Institute of Vicenza (IRRIV), San Bortolo Hospital, Vicenza, Italy • Published: February 11 , 2015

Unmet Needs in Infectious Disease • Antiviral Drug - R esistance – HIV & HCV • Latent Virus Activation – Immune Suppression • HIV, Organ Transplants, Sepsis • Bioterror & Pandemic Threats – Leading broad - spectrum countermeasure against known and unknown viruses
BIOTERROR & PANDEMIC VALIDATIONS Ebola Pandemic Influenza West Nile Pox Viruses Lassa Dengue

ONE MORE THING

The A e thlon Hemopurifier®

CANCER SAVE LIVES
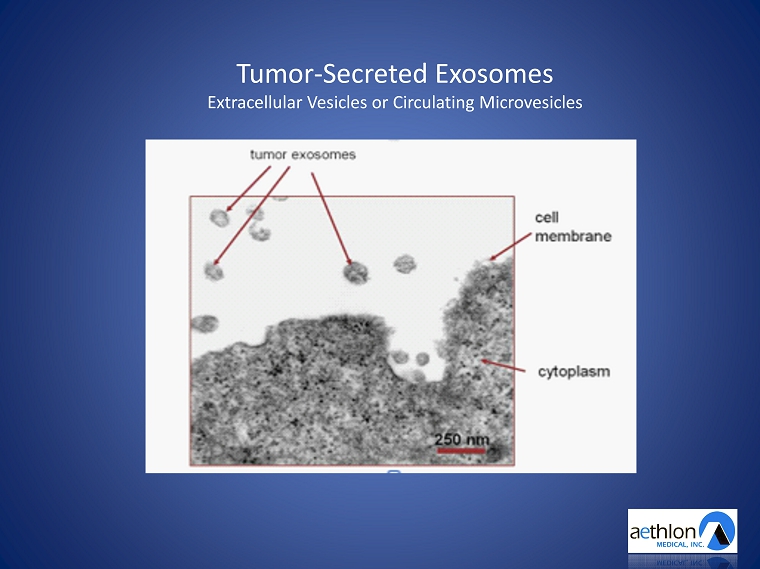
Tumor - Secreted Exosomes Extracellular Vesicles or Circulating Microvesicles

May, 2014 Trends in Molecular Medicine "Extracellular Vesicles: Emerging Targets for Cancer Therapy"
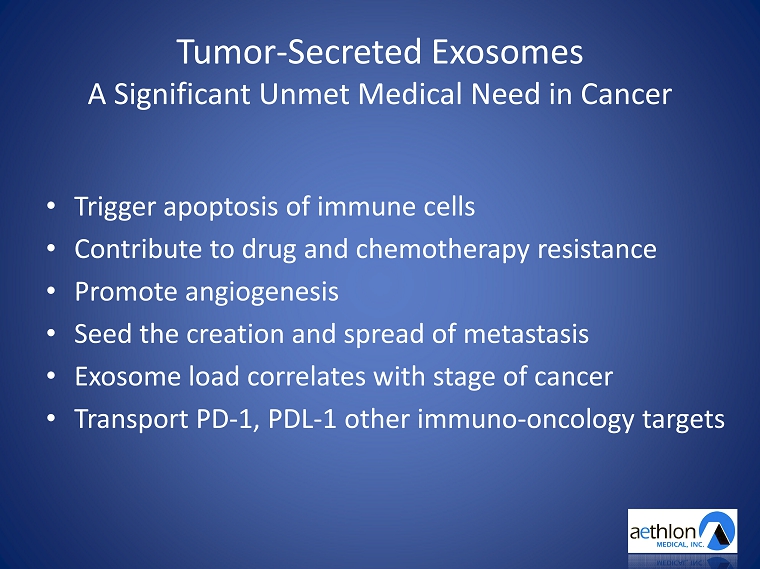
Tumor - Secreted Exosomes A Significant Unmet Medical Need in Cancer • Trigger apoptosis of immune c ells • Contribute to drug and chemotherapy resistance • Promote angiogenesis • Seed the creation and spread of metastasis • Exosome load correlates with stage of cancer • Transport PD - 1, PDL - 1 other immuno - oncology targets

An Adjunct Immunotherapeutic Device

Selected Clinical & Corporate Goals • Complete FDA Approved IDE Study – Prep for pivotal study • Advance HUD submissions – Disease conditions affecting < 4000 per yr. in the U.S. • Complete NASDAQ Up - listing • Launch Immuno - Oncology Programs

THERAPEUTIC BIOFILTRATION DEVICES TO ADDRESS LIFE - THREATENING DISEASES

SAVE LIVES