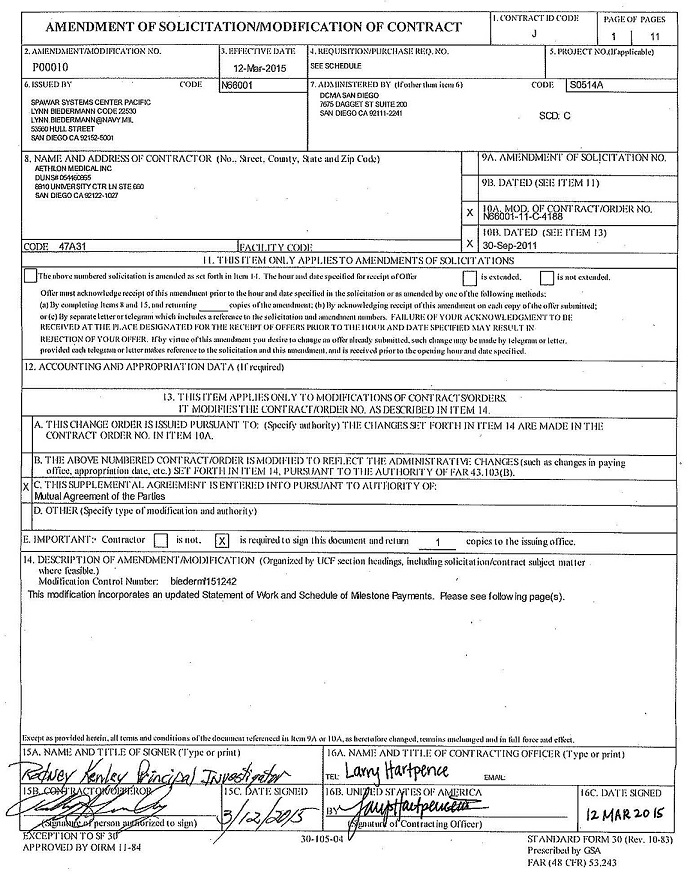
DARPA CONTRACT MODIFICATION
Published on April 17, 2015
Exhibit 10.52
SECTION SF 30 BLOCK 14 CONTINUATION PAGE
SUMMARY OF CHANGES
SECTION C - DESCRIPTIONS AND SPECIFICATIONS
The following have been modified:
STATEMENT OF WORK (SOW)
Aethlon Medical, Inc.
DATE: 11 December 2013, Revised: 12 March 2015
TITLE: Broad Spectrum Countermeasures for Viral and Bacterial Sepsis using Dialysis-Like Devices
1.0 Scope
The scope of this effort is to use Aethlon’s Hemopurifier as the core technology within an extracorporeal blood purification device that would simultaneously remove: viruses, virally-derived immunosuppressive glycoproteins, and multiple classes of exosomes; complement activation, activation of virus growth (e.g. cytomegalovirus) and TLR activation, all of which have implications to the promotion of the well-being and recovery of wounded warfighters and the prevention of sepsis.
1.1 Introduction
This effort will create an adaptable dialysis-like platform (ADAPT) technology that allows for the selective removal of harmful agents from the entire circulatory system. This revolutionary advance overcomes the limitation of devices that indiscriminately adsorb or solely capture particles by molecule size. The platform will provide an expansive therapeutic filtration mechanism to immobilize multiple affinity agents directed toward precursors to sepsis, bacterial toxins, viral pathogens, and disease enhancing particles transported by exosomes. To insure benefit to wounded warfighters, this effort will advance an innovative strategy that will allow therapy administration without systemic anticoagulation.
The ADAPT platform has been shown previously to create a broad-spectrum antiviral device that immobilized one lectin affinity agent, resulting in the effective capture of all tested Category A pathogens, as well as exosomes underlying tuberculosis and cancer. In human studies, this same device, known as the Hemopurifier, consistently provided greater than 50% average viral load reductions during four-hour treatment periods in both hepatitis-C and HIV infected individuals without antiviral drug therapy.
The resulting device would save thousands of military and civilian lives each year. Each of these technology advancements will be integrated into a single cartridge that will provide decision-free and life-saving medical care for the wounded warfighter.
1.2 Background
The goal of the DLT program is to develop a portable device that removes “dirty” blood from the body, separates harmful agents, and returns “clean” blood to the body in a manner similar to dialysis treatment of kidney failure. While the device could have an impact across multiple areas of medicine, the target application for this device is sepsis. The envisioned device will persistently interrogate the entire blood volume, providing early identification of the presence of a pathogen. Once the presence of pathogens has been confirmed, the DLT device will provide continuous "label-free" removal of pathogens, toxins and activated patient cells without pathogen identification or use of pathogen-specific binding chemistries. As a final step in the treatment process, the DLT device will enable closed-loop therapy based on continuous, reduced dimensionality modeling of patient health. Predictive modeling in this fashion will allow us to identify sepsis early, learn what we need to remove, and direct the most effective intervention to improve patient health. This cycle of sensing, adjustment, estimation, computation, and manipulation will modulate key health parameters faster than the underlying disease process and drive the patient towards a stable, healthy state.
| 2 |
2.0 Technical Requirements
2.1 Human and Animal use
Human use is anticipated in this effort, specifically related to the use of human blood. The grantee shall obtain all necessary Institutional Review Board (IRB) approvals, show proper assurance documentation, and obtain proper approval from the Government officials prior to human use testing. Funds associated with human subjected testing shall not be released until IRB documentation has been provided to SSC's 1-IRPO and approval to release funds has been obtained.
Animal use is anticipated in this effort. The contractor shall obtain all necessary Institutional Animal Care and Utilization Committee (IACUC) approval and demonstrate this approval to the Government (both ACURO and SSC-Pacific) prior to beginning experimentation with animals. If animal use is no longer anticipated, or changes significantly from the approved IACUC then the Principal Investigator (PI) must submit a letter stating the discontinuation of animal use for this effort and/or receive appropriate authorization for IACUC changes of previously specified protocols. Unless prior approval by DARPA is given IACUC documentation must be provided prior to contract award.
2.2 Base Effort (Year 1)
2.2.1 Subtask la: Anticoagulant-free Hemopurification Device
2.2.1.1 Write requirements definition for the extracorporeal blood purification system and acquire necessary equipment.
2.2.1.2 Fabricate breadboard prototypes for anticoagulation-free anti-sepsis extracorporeal system (ASEPSYS) device. Fabricate prototype blood tubing sets. Acquire anti-thrombogenic surface-modified hollow fiber plasma separators.
2.2.1.3 Assemble and test breadboard ASEPSYS devices ex vivo with bovine blood. The test will most likely be conducted using a porcine model where the elapsed time to reach a pre-defined degree of clotting in the blood treatment device will be compared between the new device and two control groups; one using standard anticoagulant therapy and one using none. Determine contribution of the following techniques and a..roaches to eliminating anticoagulants:
[***]
2.2.1.4 IRB Documentation Generation: The contractor shall obtain all necessary IRB documentation and obtain both institutional and Government (SSC-Pacific) approval in accordance with IRB documentation submission guidance prior to conducting human subject testing.
Milestones
| Ml: | Demonstrate the effectiveness of the prototype device in preventing platelet
activation or clotting in at least a 2 hour blood pumping experiment at 100 mL/hr blood flow. |
2.2.2 Subtask 2: Removal of Sepsis Precursors
2.2.2.1 Begin to develop a device based on Aethlon's ADAPT system to efficiently capture sepsis precursors identified as potentially important in killing patients undergoing sepsis. The strategy is takes advantage of the flexibility and rapidity of modification of our ADAPT platform system to test any sepsis precursor candidates that circulate in the blood. The sepsis precursors that will be targeted are shown in Table I, in order of importance. No test for the removal of bacterial toxins. No testing for removal of cytokines, since the evidence to date does not support a role for them in death due to sepsis. Additional factors may become known during the grant period and those will also be tested as time and budget permit.
[***] This material has been omitted pursuant to a request for confidential treatment and filed separately with the Securities and Exchange Commission.
| 3 |
2.2.2.2 Screening Capture Agents: Perform initial screening of the different proposed capture agents by measuring binding affinity and kinetics using surface plasmon resonance (SPR) or biolayer surface interferometry (BLI).
2.2.2.3 Perform quantitative real time PCR will also be used to measure viral load, and specific DNA or RNA targets.
Milestones
M2: Target capture > 50% in 24 hours for at least 1 target in blood or blood components
Table I Potential Target Sepsis Precursors and Broad Spectrum Binding Agents
| Septic Exosomes | Removal Agent |
| INOS exosomes | GNA |
| Viruses Associated with Sepsis | |
| Dengue Fever virus | GNA |
| H1N1 Flu virus | GNA |
| Cytomegalovirus ** | GNA |
| Herpes viruses – e.g. HSV 1 | GNA |
| Poxviruses - e.g. Vaccina (model for small pox) | GNA |
| Activation of Innate Immunity | |
| LPS endotoxins | GNA |
| LTA endotoxins | GNA |
2.3 Option 1 (Year 2)
2.3.2 Subtask 1a: Anticoagulant-free Hemopurification Device
2.3.2.1 Demonstrate the effectiveness of the prototype device in vivo in animals preventing platelet activation or clotting in at least a 2 hour blood pumping experiment at 75 mL/min blood flow.
2.3.2.2 Formulate initial design based on work from previous phase. Begin to build and test selected instrument design and tubing sets.
2.3.2.3 Write and test software. Conduct ergonomic research. Begin discussions with System Integrator.
2.3.2.4 Complete fabrication and testing of prototype instrument.
2.3.2.5 Research literature and patent database for technical options for an on-line/in-line sensor with the ability to quantify the clearance of citrate and/or calcium in effluent dialysate.
2.3.2.6 Collect feasibility data on at least one sensor option.
2.3.2.7 Conduct a series of 21 experiments aimed at characterizing the contribution of several alternate fluidic designs and methods of perfusing plasma filters and affinity columns in the performance of affinity plasmapheresis.
2,3.3 Subtask 2. Removal of Sepsis Precursors
2.3.3.1 Build the ADAPT capture cartridges with the identified affinity agents. Measure the rate of capture of the specific targets from in ex vivo recirculation experiments from cell culture and blood.
2.3.3.2 Cartridge construction with optimized affinity matrix design for each potential target. Complete all capture agents screening. Initiate ex vivo capture studies from blood using the optimized cartridges.
Milestones
M4: Target capture > 50% in 24 hours for at least 5 targets in blood or blood components.
M5: Milestone 5: Target capture > 90% in 24 hours for at least 3 targets in blood or blood components.
NOTE: TASK 2.3.2 SHALL NOT BE EXERCISED AND TASKING FUNDS RELEASED UNTIL IRB DOCUMENTATION AND PROPER IRB APPROVAL HAS BEEN OBTAINED.
2.4 Option 2 (Year 3)
| 4 |
2.4.1 Subtask 1a: Anticoagulant-free Hemopurification Device
2.4.1.1 Design and fabricate optimized configuration(s) of hemopurification device(s) that contain(s) a combination of hemofilters, plasma filters, and affinity columns.
Milestones
| M6: | Define Aethlon’s GMP manufacturing
process and revise and upgrade Aethlon’s quality procedures and policies to the
current state of the art. |
2.4.2 Subtask 4: Target Capture in Combined Agent Cartridge
2.4.2.1 Evaluate contribution of manufacturing process variables to binding capacity of affinity resin.
2.4.2.2 Determine capacity requirements of affinity resin to multiple simultaneous targets.
2.4.2.3 Perform basic biocompatibility tests for the combination ADAPT device to confirm the combination cartridge does not present additional risk.
2.4.2.4 Finish construction and delivery of 25 experimental cartridges for testing by the system integrator.
Milestones
M7: Target capture > 90% in 24 hours (12 months) for at least 3 targets ex vivo in blood or blood components using the optimized cartridge.
M8: Pass biocompatibility tests for the combination ADAPT device.
M9: Construct and deliver of 25 prototype cartridges for testing by the system integrator.
2.5 Option 3 (Year 4)
2.5.1 Subtask 1a: Anticoagulant-free Hemopurification Device
2.5.1.1
Complete Aethlon’s GMP procedure and establish and maintain all GMP documentation for the company.
Revise and upgrade Aethlon’s quality procedures and policies to the current state of the art and establish and maintain
all GMP documentation for the company.Develop additional optimized configuration(s) of hemopurification device(s) that contain(s)
a combination of hemofilters, plasma filters, and affinity columns.
Milestones
M11:
Develop a strategic plan for developing an alternate method of producing GNA by cloning the gene into
an alternate vector and identify potential partners for such production. Demonstrate the safety of an additional prototype
device within an optimized fluidic circuit architecture preventing clotting in a 24 hour experiment in vivo at a blood
flow rate of 200 ml/min using either pigs or dogs.
2.5.2 Subtask 4: Target Capture in Combined Agent Cartridge
2.5.2.2 Finish construction and delivery of 50 prototype cartridges for testing by the system integrator. The cartridges will need to be made available (packaged, labeled, sterilized and qualified) to the system integrator.
Milestones
M13: Construct and deliver of 50 prototype cartridges for testing by the system integrator.
2.6 Option 4 (Year 5)
2.6.1 Subtask 5: Testing of final product by System Integrator
2.6.1.1 System integrator acceptance of the hemofilter device.
2.6.1.3 Prepare and submit IDE proposal for sepsis treatment.
2.6.1.4 Prepare and present Final report for DARPA.
| 5 |
Milestones
M12: System Integrator approval of a sepsis precursor ADAPT treatment cartridge
3.0 Program Management and Reviews
3.1 Program Management Plan
The contractor shall develop a Program Management Plan. A graphical representation of this plan (Gantt chart is one example) identifying major tasks and their task leaders, milestones of the major task and their completion dates shall be generated. In addition, a graphical representation of budget shall be generated.
3.2 Kick-off Meeting
The contractor shall participate in a kick-off meeting within 60 days of contract award. The purpose of this meeting is to introduce key program personnel, discuss the proposed tasking, present the program schedule and milestones and the initial Program Management Plan.
3.3 Quarterly Reviews
The contractor shall hold quarterly reviews for the duration of this effort. The purpose of these reviews is to present a summary of work completed and milestones met, discuss any problems encountered, update the program schedule, present the program financial status, and discuss remaining work.
3.4 Final Contract Review
A final contract review held in place of the last quarterly review shall be hosted by the principal contractor. The purpose of this review is to present a summary of all work completed and milestones accomplished and to discuss any relevant future efforts similar to the contract that may be pursued.
4.0 Deliverables
The reports and presentation materials are to be delivered in accordance with the attached Data Delivery Matrix.
(End of SOW).
SECTION G - CONTRACT ADMINISTRATION DATA
The following have been modified:
SCHEDULE OF PAYMENT MILESTONES
12 month duration base period (Year 1)
| Milestone | Month | Payable Milestone |
GOVT CONTRIBUTION |
| Subtask 1a | Anticoagulant-free Hemopurification Device | ($908,384) | |
| 2.2.1.1 | 1 | Write requirements definition for the extracorporeal blood purification system and acquire necessary equipment. | ($358,284) |
| 6 |
| 2.2.1.2 | 3 | Fabricate breadboard prototypes for anticoagulation-free anti-sepsis extracorporeal system (ASEPSYS) device. Fabricate prototype blood tubing sets. Acquire anti- thrombogenic surface-modified hollow fiber plasma separators. | ($183,367) |
| 2.2.1.3 | 6 | Assemble and test breadboard ASEPSYS devices. Evaluate the use of different techniques and approaches to eliminating anticoagulants. | ($183,367) |
| 2.2.1.4 | 12 | Obtain all necessary IRB documentation and obtain both institutional and Government (SSC-Pacific) approval in accordance with IRB documentation submission guidance prior to conducting human or animal testing. | ($183,367) |
|
Subtask 2 & 4 |
Removal of Sepsis Precursors |
($1,066,663) | |
| 2.2.2.1 | 2 | Begin to develop the Aethlon’s ADAPT device to efficiently capture sepsis precursors and acquire important equipment and supplies | ($416,424) |
| 2.2.2.2 | 5 | Perform initial screening of the different proposed capture agents by measuring binding affinity and kinetics using surface plasmon resonance (SPR) or biolayer surface interferometry (BLI). | ($216,747) |
| 2.2.2.3 | 8 | Perform preliminary quantitative real time PCR to measure viral load, and specific DNA or RNA targets. | ($216,747) |
| M2 | 12 | Target capture > 50% in 24 hours for at least 1 target in blood or blood components | ($216,747) |
| Total | ($1,975,047) |
| 7 |
12 month duration option #1 period (Year2)
| Milestone | Month | Payable Milestone |
GOVT CONTRIBUTION |
| Subtask 1a | Anticoagulant-free Hemopurification Device | ($782,322) | |
| 2.3.2.1 | 15 | Demonstrate the effectiveness of the prototype device in vivo in animals preventing platelet activation or clotting in at least a 2 hour blood pumping experiment at 75 mL/min blood flow. | ($195,581) |
| 2.3.2.2 | 18 | Formulate initial design based on work from previous phase. Begin to build and test selected instrument design and tubing sets. | ($195,581) |
| 2.3.2.3 | 21 | Write and test software. Conduct ergonomic research. Begin discussions with System Integrator. | ($195,581) |
| M3 | 24 |
Complete fabrication and testing of prototype Research literature and patent database for technical. |
($195,581) |
| Subtask 2 | Removal of Sepsis Precursors | ($835,124) | |
| 2.3.3.1 | 15 | Build the ADAPT capture cartridges with the identified affinity agents. Measure the rate of capture of the specific targets from in ex vivo recirculation experiments from cell culture and blood. | ($208,781) |
| M4 | 18 | Target capture > 50% in 24 hours for at least 5 targets in blood or blood components. | ($208,781) |
| 2.3.3.2 | 21 | Cartridge construction with optimized affinity matrix design for each potential target. Complete the capture agent screening. | ($208,781) |
| M5 | 24 | Target capture > 90% in 24 hours for at least 3 targets in blood or blood components. | ($208,781) |
| Total | ($1,617,446) |
| 8 |
12 month duration option #2 period (Year 3)
| Milestone | Month | Payable Milestone |
GOVT CONTRIBUTION |
| Subtask 1a | Anticoagulant-free Hemopurification Device | ($372,328) | |
| 2.4.1.1 | 27 | Design and fabricate optimized configuration(s) of hemopurification device(s) that contain(s) a combination of hemofilters, plasma filters, and affinity columns. | ($186,164) |
| M6 | 36 | Define Aethlon’s GMP manufacturing process and revise and upgrade Aethlon’s quality procedures and policies to the current state of the art. | ($186,164) |
|
Subtask 2+4 |
Target Capture in Combined Agent Cartridge | ($720,726) | |
| 2.4.2.1 | 27 | Evaluate contribution of manufacturing process variables to binding capacity of affinity resin. | ($197,362) |
| 2.4.2.2 | 33 | Determine capacity requirements of affinity resin to multiple simultaneous targets. | ($197,362) |
| 2.4.2.3 | 30 | Perform biocompatibility tests for the combination ADAPT device to confirm the combination cartridge does not present additional risk. | ($78,641) |
| 2.4.2.4 | 36 | Finish construction and delivery of 25 experimental cartridges for testing by the system integrator. | ($50,000) |
| M9 | 36 | Target capture > 90% in 24 hours (12 months) for at least 3 targets ex vivo in blood or blood components using the optimized cartridge. | ($197,361) |
| Total | ($1,093,054) |
| 9 |
12 month duration option #3 period (Year 4)
| Milestone | Month | Payable Milestone |
GOVT CONTRIBUTION |
| Subtask 1a | Anticoagulant-free Hemopurification Device | ($276,172) | |
| 2.5.1.1 | 45 | Complete Aethlon’s GMP procedure and establish and maintain all GMP documentation for the company. | ($90,008) |
| M11 | 48 | Develop a strategic plan for developing an alternate method of producing GNA by cloning the gene into an alternate vector and identify potential partners for such production. | ($186,164) |
|
Subtask 2+4 |
Target Capture in Combined Agent Cartridge | ($296,964) | |
| 2.5.2.2 | 48 | Finish construction and begin delivery of 50 prototype cartridges for testing by the system integrator. | ($296,964) |
| Total | ($573,136) |
| 10 |
12 month duration option #4 period (Year 5)
| Milestone | Month | Payable Milestone |
GOVT CONTRIBUTION |
| Subtask 5 | Testing of final product by System Integrator | ($581,157) | |
| 2.6.1.1 | 51 | System integrator acceptance of the hemofilter device. | ($193,719) |
| 2.6.1.3 | 57 | Prepare and submit IDE proposal for sepsis treatment. | ($193,719) |
| 2.6.1.4 | 60 | Prepare and present Final Report for DARPA. | ($193,719) |
| Total | ($581,157) |
(End of Milestone Schedule)
(End of Summary of Changes)
| 11 |