PROTOCOL FOR UCI CLINICAL TRIAL
Published on April 15, 2015
Exhibit 10.2
Plasma Exosome Concentration in Cancer Patients Undergoing Treatment
Objectives of the Study:
This is a pilot study of a putative biomarker, circulating exosomes, which are increased in patients with cancer and hypothesized to play a role in tumor-associated immune suppression. We will address the following Specific Aims:
| 1) | Characterize the baseline concentration of circulation exosomes in patients with various tumor types (in and around the time of diagnosis) and the kinetics of longitudinal changes in the circulating exosome concentration associated with primary therapy (surgery, radiation therapy, chemotherapy, and/or neoadjuvant chemotherapy). |
| 2) | In patients with metastatic tumor burden, examine baseline and longitudinal changes associated with chemotherapy treatment. |
| 3) | In patients with metastatic tumor burden evaluate associations between changes in circulating exosome concentrations and response to chemotherapy. |
RESEARCH DESIGN AND METHODS
Overview of Study Design
This proposed clinical study will provide critical initial data to direct future clinical investigations of a novel treatment approach using extracorporeal hemofiltration for the removal of tumor-derived exosomes. Exosomes are small, 30-120 nm, membrane bound vesicles shed from all cells, but at higher levels from tumor cells [1], providing a logical candidate for the association between tumor burden and tumor-associated immune suppression. First described > 30 years ago, exosomes were originally thought to be cellular “garbage bags,” found in the peripheral blood, containing proteins (including various receptors and signaling molecules) and nucleic acids, (genomic, mRNA, and microRNAs) [1, 2]. Exosomes are now recognized to have many biological functions including expressing tumor associated antigens [3-7], to tumor-associated immune suppression [8-19], and have been hypothesized to directly contribute to the metastatic process and treatment resistance, but with mechanisms less well characterized [8, 12, 20-23]. It has been observed that circulating exosome concentration is increased in patients with metastatic melanoma, ovarian epithelial, prostate and colorectal neoplasms [24-29] and as such is hypothesized to vary with tumor burden.
Exosome ELLSA:
Previous studies have established that tumor- secreted exosomes display high mannose glycoprotein signatures on their surfaces [30], thus sharing this feature in common with enveloped viruses. To this end, Aethlon has examined exosomes from various tumor sources and tested their binding to GNA affinity matrices, in vitro, in studies with Dr. Douglas Taylor. In a variation of a traditional ELISA, GNA was used as a substrate for coating plates to measure exosome binding, enzyme linked lectin sorbent assay (ELLSA). Ovarian cancer exosomes that were purified by a conventional ultracentrifugation method bound to GNA and were detectable against a mannan standard curve, Figure 1.
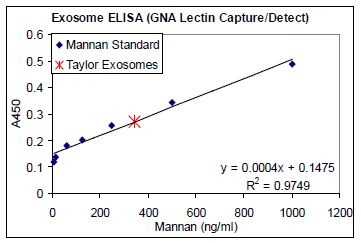
FIGURE 1. Exosome ELLSA. Binding of purified exosomes from an ovarian cancer patient (obtained by ultracentrifugation) to GNA coated plates (1 μg/mL) in a modified ELISA assay using serial dilutions of mannan and HRP labeled GNA as a detection agent to generate a standard curve.
| 1 |
Aethlon’s initial studies were conducted by Dr. Douglas Taylor using exosomes from ovarian cancer patients. Exosomes were purified from ascites fluid of two ovarian cancer patients using an established ultracentrifugation method [31, 32] or using the Hemopurifier®. The Hemopurifier® provides the capacity to elute adsorbed exosomes for subsequent evaluation. Analysis of the tumor-associated exosome marker, EpCAM, revealed that the presence of EpCAM in the material eluted from the Hemopurifier® as well as in the control exosome preparation, Figure 2 Panel A.
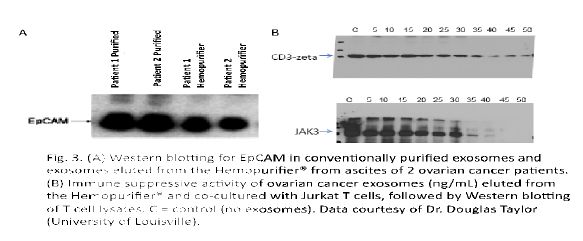
FIGURE 2. Panel A: Western blot for EpCam in conventionally purified exosomes and exosomes eluted from the Hemopurifier from ascites of 2 cancer patients. Panel B: Effect of co-culture of eluted exosomes at increasing concentrations with Jurkat T cells followed by western blotting of cell lysates for CD3 zeta chain and phospho JAK3. C=control (no exosomes).
Subjects and Recruitment:
Subject Recruitment/Informed Consent: Recruitment of participants in the study will be through the use of internal and outside referrals to the University of California, Irvine (UCI) Medical Center (UCIMC). Review and approval of this human protocol will be conducted by the UCI Institutional Review Board (IRB) Human Subjects Review Committee. Subjects must be able to understand and sign an informed consent form, which must comply with U.S. regulations (U.S. 21 CFR 50) and ICH guidelines to be eligible for this trial. All study materials, including consent forms will be provided in the patient’s primary language. In addition, each subject will be given a copy of the consent form, the Experimental Subjects’ Bill of Rights, and HIPAA Release form, all of which will be explained to the subject.
Accrual Capacity: The NCI designated Chao Family Comprehensive Cancer Center (CFCCC) at UCI has an active clinical cancer care and research program. UCI Medical Center has a large referral base and sufficient numbers of patients will be available for enrollment onto the protocol described herein. Data from the cancer registry documents that over the past three years, we have seen a steady rise from our baseline of approximately 1700 individual new “analytic” patients: 2010 = 1697, 2011 = 2000, 2012 = 2164, 2013 = 2189 (with 257 of these being new cases with metastatic disease) and are on track for a further increase in 2014. We have an outstanding record of accrual to clinical trials with over 24% of patients being accrued to clinical studies in each of these three years; the national average at NCI designated comprehensive cancer centers is 15% and nation wide is only 5%. Even given a conservative 30% accrual rate of screened and eligible patients and not accounting from recruitment from the larger surrounding non-UCI affiliated oncology care community, we have adequate capacity to successfully complete accrual to this study.
| 2 |
Study Population Demographics: Those who satisfy the inclusion/exclusion criteria and will be enrolled in the study. Patient enrollment will include all ethnic groups and both genders as available. The current demographics of the patient population seen at CFCCC and the University of California at Irvine Medical Center are as follows: 42% male; 59% female; 45%Caucasian; 5% Black; 20% Asian; 30% Hispanic.
Study Endpoints
A. Baseline circulating exosome concentration
B. Longitudinal changes in circulating exosome concentrations associated with tumor treatment.
C. Association of longitudinal changes in circulating exosome concentrations with response to treatment.
Evaluation of clinical outcome per se, (i.e., progression free or overall survival) is beyond the scope of this pilot study, we will explore potential associations with clinical outcomes and the putative biomarker, circulating exosome concentration which will provide additional pilot data to direct future studies.
Study Population - Patient Inclusion and Exclusion Criteria
Patients with a diagnosis of cancer are potential subjects for this study.
In order to be eligible for this study, patients must meet all of the following criteria:
1. Patients must have a histologically proven diagnosis of cancer.
2. Have measurable tumor burden.
3. Expected survival must be greater than six (6) months.
4. A Karnofsky Performance Status (KPS) must be 70 or greater, equivalent to ECOG 0 to ≈2 (Appendix A).
5. Patients must be >21 years of age.
6. Patients must be able to understand and sign an informed consent form, which must comply with U.S. regulations (U.S. 21 CFR 50) and ICH guidelines.
Patients with any one of the following conditions must be excluded from this trial:
1. History of repeated central line associated thrombosis or bleeding diathesis.
2. Chronic anti-coagulation therapy.
3. More than one malignant diagnosis, except for the basal cell epithelioma of the skin.
4. Persistent fever greater than 38 C.
5. Calculated CrCl less than 60 ml/min.
6. Required use of chronic corticosteroids or immune suppression for any reason including an organ allograft or HIV infection
7. Patients with any acute or chronic illness including cardiovascular disease or history of myocardial infarction, autoimmune state, or any psychiatric illness that in the opinion of the Investigators would compromise treatment.
Study Design/Sample Size:
As this is a pilot, hypothesis generating early phase non-randomized, clinical study, designed to provide initial characterization of a tumor biomarker, and is not a hypothesis testing Phase II or III study, standard calculations of “power” or “sample size” are not appropriate or applicable for this study. It is not known if there are significant differences in circulating exosome concentrations by tumor type, treatment type, or kinetics of treatment response. Thus, the final target study cohort size will be dependent upon early observations in a range of subjects from various tumor types. We will employ cohort expansion modifications as indicated. We initially plan on enrolling 5 patients with defined tumor types, see table next page, for a total initial study population of 45 subjects.
| 3 |
If analysis of the initial 5 subjects with a given tumor type suggests impact of histologic subtype (e.g. for breast cancer – hormone receptor positive, HER2/neu positive, triple negative, lobular vs. ductal), treatment type or kinetics of treatment response; expansion cohorts consisting of 5 subjects will be added, via modification of the IRB approved protocol, as needed to characterize the impact of these factors on circulating exosome concentrations.
| Tumor Type | Study Cohort |
Approximate number of cases at UCIMC on treatment with tumor present - 2013 |
| Breast adenocarcinoma | 5 | 30 |
| Colorectal | 5 | 21 |
| Gastric & Gastroesophageal | 5 | 30 |
| Pancreatic | 5 | 60 |
| Cholangiocarcinoma | 5 | 9 |
| Lung (NSCLC) | 5 | 44 |
| Head & Neck (SCC) | 5 | 42 |
| Melanoma | 5 | 22 |
| Ovarian adenocarcinoma | 5 | 23 |
Research Methodology/Study Procedures
Enrolled subjects will have standard phlebotomy performed with collection of blood into 4 ml vacutainer tubes containing K2EDTA anticoagulant. These tubes will be transported to Dr. Nelson’s laboratory at 4 C (in the presence of a cool pack) for centrifugation at 300 g (≈ 1200 RPM for tabletopcentrifuge - Beckman GH-3.8). Plasma will be removed from the vacutainer tube and distributed as 1 ml aliquots into 1.5 ml eppendorf snap top tubes labeled with the subjects 4 digit study ID number (e.g. 1001, cohort 1, subject #1). These tubes will be placed at -80 C until shipment on dry ice to Princeton NJ, for assay of exosome concentration.
Subjects will have phlebotomy performed immediately before the administration of treatment, primary treatment or treatment for advanced disease. This blood sample will be collected prior to the administration of any pre-medications for subsequent treatment, (surgery, chemotherapy, chemoradiation, or radiation alone, the latter will be rare). Each sample will be labeled to identify the patient as with any other biospecimen collected for clinical evaluation. Ideally, the first sample will be collected before any treatment has been initiated, however there is no absolute requirement that the patients have not received prior treatment if they have advanced disease. For those patients continuing treatment but with measurable, advanced disease, progression free survival and time to treatment failure will have less meaning and we hypothesize, will be less likely to demonstrate associations with circulating exosome concentrations. Never the less, the first collection will be considered the patient’s baseline value. For multiple day regimens, patients will have 4 ml of blood collected on the first and last day of the chemotherapy regimen. Additional 4 ml samples will be collected no more frequent than weekly during the first four weeks of enrollment on this protocol. Thus, regardless of whether treatment is primary (for a new diagnosis and therefore potentially primary surgical treatment), is neoadjuvant, or is secondary for advanced disease the subjects will get a minimum of 4 x 4 ml phlebotomy samples collected in the first month. Thereafter, phlebotomy will be performed with each cycle of chemotherapy (neoadjuvant or secondary) or with each post primary treatment follow-up visit for no more than 6 months from the baseline collection.
| 4 |
Endpoint Data and Evaluations:
Circulating Exosome Concentrations:
Samples of plasma shipped frozen to Princeton NJ, will be analyzed in replicate via the ELLSA assay described above, quantifying the concentration of circulating exocomes in each sample. Raw and mean data will be provided for data analyses. Grubbs test will be used to identify outliers from triplicate or larger replicate determinations of exosome concentration from each sample.
Other Data to be Collected:
Treatment and Response: Patients may be receiving so called “targeted therapies”, standard cytotoxic chemotherapy, combined chemoradiation, and/or symptomatic/palliative support without cytotoxic therapies. Select agents can induce different types of tumor cell death and may have different effects on the release of tumor-associated exosomes. It is conceivable that an immediate and profound cytotoxic effect may lead to a transient “bolus” release of tumor-associated exosomes. Thus, treatment administered will be collected to investigate the impact of particular agents or combinations thereof, on the longitudinal changes in circulating exosome concentration. Additionally, we will collect tumor response data from the clinician’s notes to assess the association between changes in tumor burden and the longitudinal changes in circulating exosome concentrations.
Progression Free Survival and Time to Relapse: All patients will be followed from the time of enrollment for PFS and TTR for a maximum of five years. These clinical parameters will be used to investigate potential associations with longitudinal changes in the circulating exosome concentration.
Sociodemographic Characteristics: Race and ethnicity will be recorded for all participants in order to ascertain whether these impact circulating exosome concentration. This will inform the design of future studies. In addition, the use of prescription and nonprescription medications will be recorded as these may also confound the circulating exosome concentration.
Cancer & Medical History: Diagnosis, prior treatment, comorbidities, as well as histology classification will be recorded. Social history including education level, activity level, will also be recorded to investigate potential confounding co-variables.
Statistical Considerations & Data Analysis
Descriptive statistics will be computed to summarize demographic and background variables, efficacy variables and toxicity variables. Comparisons of baseline characteristics will be performed using univariate analysis of variance for continuous variables and Chi-square analyses for categoric variables. Changes over time in circulating exosome concentrations and associations with other categoric variables will be evaluated by multivariate analysis of variance methods for repeated measures. Because this is an early phase study, the sample size is not based on the power necessary to test a hypotheses for a given or expected effect size.
Risk
The risk to the subjects is limited to the risk of phlebotomy and the risk of breach of HIPAA security Phlebotomy Risk: Collection of blood during the phlebotomy visit may cause pain, brief dizziness, possible fainting, slight bleeding, swelling or bruising at the collection site, and a very remote chance of infection. To minimize this risk certified and experienced phlebotomists or RNs will collect the specimens.
| 5 |
HIPAA Risk: We recognize that there is a risk for confidential information to be exposed. Biomarkers are stored in Dr. Nelson’s laboratory. Clinical data is maintained within the EMR and if transferred to any desktop computer, that computer will be maintained in a locked office, encrypted and password protected in accordance with University of California policies. Access to the computers and files is restricted by a high security system through password. Within our secure network, data will only be accessible to the study personnel who have signed confidentiality agreements, taken online IRB tutorials for the protection of human subjects and taken the Health Insurance Portability and Accountability Act online tutorial. Computers are located in offices where office doors are closed and locked after working hours and during weekends. All office doors are locked after working hours and during weekends. To additionally protect the confidentiality of participants, our central informatics system includes the following features: 1) use of encryption software to secure data transport; 2) a firewall system that protects the informatics network from intrusion or unauthorized access; 3) access to the central informatics system requires a valid password; 4) confidential data are stored in a separate secure database table and linked to data in other tables by a number only with no personal identifiers; 5) extract files and reports will not contain any confidential information.
Potential Benefits of the Proposed Research to Subjects and Others:
There will be no benefits for participants in the study.
| 6 |
References:
| 1. | Thery, C., Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep, 2011. 3: p. 15. |
| 2. | Valenti, R., et al., Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res,2 006. 66(18): p. 9290-8. |
| 3. | Schartz, N.E., et al., From the antigen-presenting cell to the antigen-presenting vesicle: the exosomes. Curr Opin Mol Ther, 2002. 4(4): p. 372-81. |
| 4. | Wolfers, J., et al., Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med, 2001. 7(3): p. 297-303. |
| 5. | Mignot, G., et al., Prospects for exosomes in immunotherapy of cancer. J Cell Mol Med, 2006. 10(2): p. 376-88. |
| 6. | Delcayre, A. and J.B. Le Pecq, Exosomes as novel therapeutic nanodevices. Curr Opin Mol Ther, 2006. 8(1): p. 31-8. |
| 7. | Hao, S., T. Moyana, and J. Xiang, Review: cancer immunotherapy by exosome-based vaccines. Cancer Biother Radiopharm, 2007. 22(5): p. 692-703. |
| 8. | Filipazzi, P., et al., Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol, 2012. 22(4): p. 342-9. |
| 9. | Whiteside, T.L., Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem Soc Trans, 2013. 41(1): p. 245-51. |
| 10. | Marton, A., et al., Melanoma cell-derived exosomes alter macrophage and dendritic cell functions in vitro. Immunol Lett, 2012. 148(1): p. 34-8. |
| 11. | Peng, P., Y. Yan, and S. Keng, Exosomes in the ascites of ovarian cancer patients: origin and effects on anti-tumor immunity. Oncol Rep, 2011. 25(3): p. 749-62. |
| 12. | Azmi, A.S., B. Bao, and F.H. Sarkar, Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev, 2013. |
| 13. | Chalmin, F., et al., Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3- dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest, 2010. 120(2): p. 457-71. |
| 14. | Xiang, X., et al., TLR2-mediated expansion of MDSCs is dependent on the source of tumor exosomes. Am J Pathol, 2010. 177(4): p. 1606-10. |
| 15. | Liu, Y., et al., Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol, 2010. 176(5): p. 2490-9. |
| 16. | Xiang, X., et al., Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer, 2009. 124(11): p. 2621-33. |
| 17. | Yu, S., et al., Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol, 2007. 178(11): p. 6867-75. |
| 18. | Clayton, A., et al., Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res, 2007. 67(15): p. 7458-66. |
| 19. | Peche, H., et al., Induction of tolerance by exosomes and short-term immunosuppression in a fully MHC-mismatched rat cardiac allograft model. Am J Transplant, 2006. 6(7): p. 1541-50. |
| 20. | Rana, S., K. Malinowska, and M. Zoller, Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia, 2013. 15(3): p. 281-95. |
| 21. | Hood, J.L., R.S. San, and S.A. Wickline, Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res, 2011. 71(11): p. 3792-801. |
| 22. | Aung, T., et al., Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci U S A, 2011. 108(37): p.15336-41. |
| 23. | Pilzer, D., et al., Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin Immunopathol, 2005. 27(3): p. 375-87. |
| 24. | Jimenez, C.R., et al., Proteomics of colorectal cancer: overview of discovery studies and identification of commonly identified cancer-associated proteins and candidate CRC serum markers. J Proteomics, 2010. 73(10): p. 1873-95. |
| 25. | Khan, S., et al., Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS One, 2012. 7(10): p. e46737. |
| 26. | Kucharzewska, P., et al., Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia- dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A, 2013. 110(18): p. 7312-7. |
| 27. | Liang, B., et al., Characterization and proteomic analysis of ovarian cancer-derived exosomes. J Proteomics, 2013. 80C: p. 171-182. |
| 28. | Logozzi, M., et al., High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One, 2009. 4(4): p. e5219. |
| 29. | Silva, J., et al., Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosomes Cancer, 2012. 51(4): p. 409-18. |
| 30. | Batista, B.S., et al., Identification of a conserved glycan signature for microvesicles. J Proteome Res, 2011. 10(10): p. 4624-33. |
| 31. | Momen-Heravi, F., et al., Current methods for the isolation of extracellular vesicles. Biol Chem, 2013. 394(10): p. 1253-62. |
| 32. | Thery, C., et al., Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol, 2006. Chapter 3: p. Unit 3 22. |
| 7 |