PRESENTATION
Published on September 23, 2014
Exhibit 99.1

JIM JOYCE CHAIRMAN, CEO EXOSOMES 2014 September 18 & 19, 2014 San Diego, California Extracorporeal Clearance of Tumor - Secreted Exosomes ; An Adjunct Strategy to Improve Cancer Treatment Outcomes
| 1 |

FORWARD LOOKING STATEMENTS The following presentation may contain predictions, estimates, and other forward looking statements that involve risks and uncertainties, including whether and when our products are successfully developed and introduced ; market acceptance of the A e thlon ADAPT™ system, the H e mopurifier ® and other product offerings ; regulatory delays, manufacturing delays, and other risks detailed in our SEC filings, which are accessible at www . sec . gov or on our website : www . A e thlonMedical . com
| 2 |

We Create Targeted Therapeutic Devices to Address Infectious Disease and Cancer
| 3 |

2005 An Accidental Journey Into The Exosome Field
| 4 |

DIAGNOSTICS THERAPEUTICS 2014
| 5 |

A “Liquid Biopsy” Organization
| 6 |

• Launched in November 2013 • Lectin - based “Liquid Biopsy” Platform • Oncology Validations – Bladder, Breast, Colorectal, Glioblastoma , Metastatic Melanoma, Ovarian, and Pancreatic Cancer • Chief Scientific Officer – Dr. Douglas Taylor
| 7 |

CANCER THERAPY
| 8 |

An Adjunct S trategy to Eliminate C irculating Tumor Exosomes W/O added drug toxicity
| 9 |
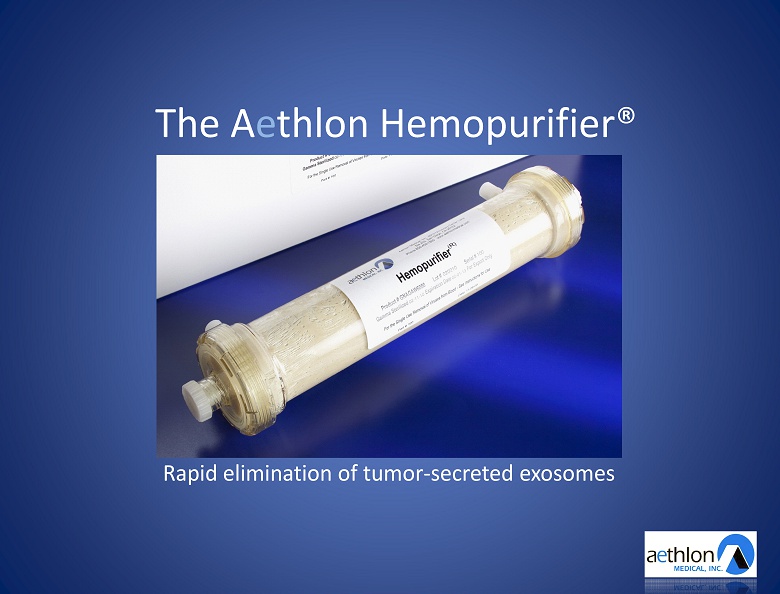
The A e thlon Hemopurifier® Rapid elimination of tumor - secreted exosomes
| 10 |
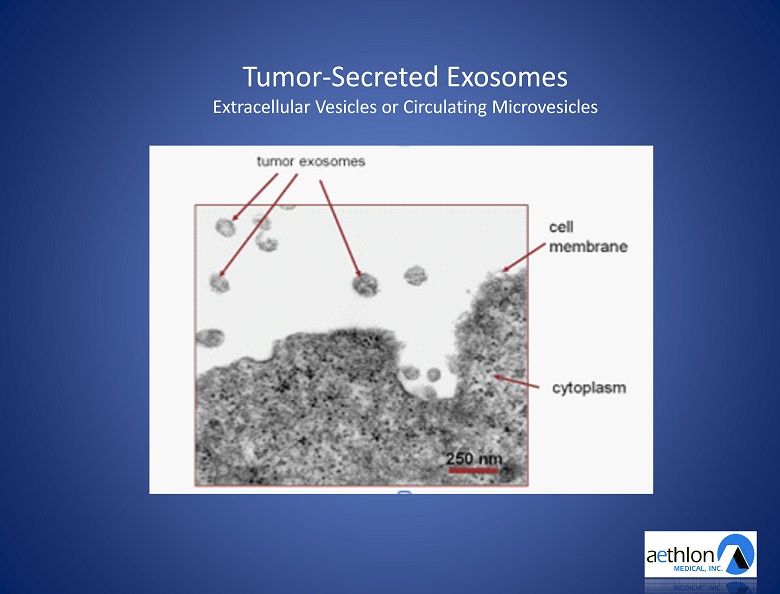
Tumor - Secreted Exosomes Extracellular Vesicles or Circulating Microvesicles
| 11 |
| 12 |
Tumor - Secreted Exosome Validations
| 13 |
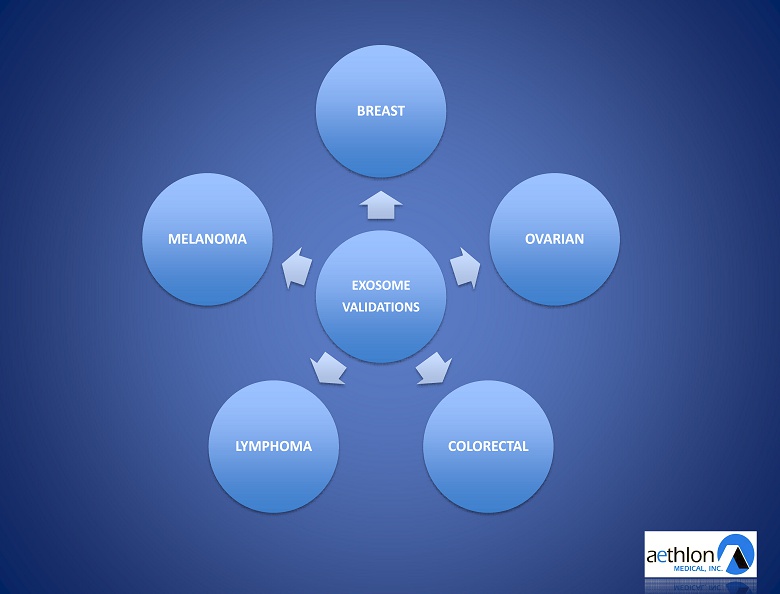
EXOSOME VALIDATIONS BREAST OVARIAN COLORECTAL LYMPHOMA MELANOMA
| 14 |

Why Target Tumor - Secreted Exosomes ?
| 15 |

May, 2014 Trends in Molecular Medicine "Extracellular Vesicles: Emerging Targets for Cancer Therapy"
| 16 |

Tumor - Secreted Exosomes • Trigger apoptosis of immune c ells • Contribute to drug and chemotherapy resistance • Promote angiogenesis • Seed the creation and spread of metastasis • Exosome load correlates with stage of cancer
| 17 |

Exosomes Transport Biopharmaceutical Industry Targets
| 18 |
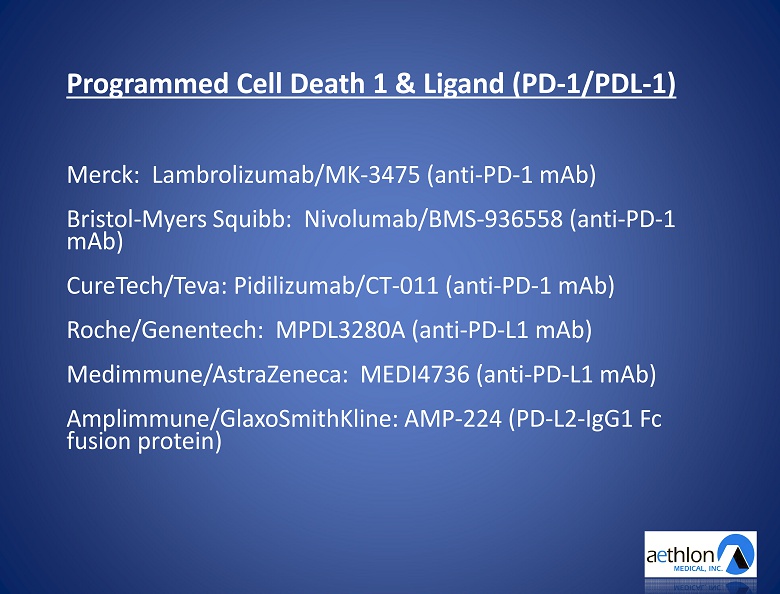
Programmed Cell Death 1 & Ligand (PD - 1/ PDL - 1) Merck : Lambrolizumab /MK - 3475 (anti - PD - 1 mAb ) Bristol - Myers Squibb: Nivolumab /BMS - 936558 (anti - PD - 1 mAb ) CureTech / Teva : Pidilizumab /CT - 011 (anti - PD - 1 mAb ) Roche /Genentech: MPDL3280A (anti - PD - L1 mAb ) Medimmune/AstraZeneca: MEDI4736 (anti - PD - L1 mAb ) Amplimmune /GlaxoSmithKline: AMP - 224 (PD - L2 - IgG1 Fc fusion protein )
| 19 |

CTLA - 4 Bristol - Myers Squibb: Ipilimumab / Yervoy ® (anti - CTLA - 4 mAb ) Medimmune /Pfizer: Tremelimumab /CP - 675 - 206 (anti - CTLA - 4 mAb )
| 20 |
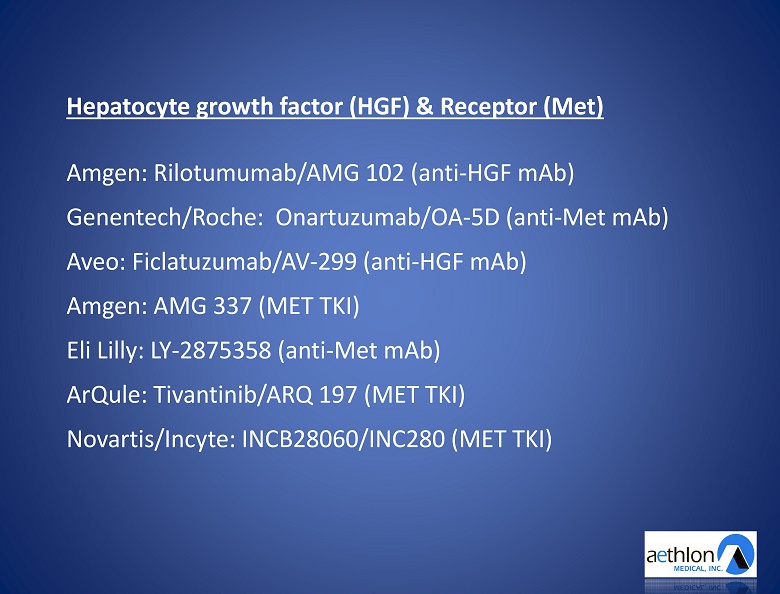
Hepatocyte growth factor (HGF) & Receptor (Met ) Amgen : Rilotumumab /AMG 102 (anti - HGF mAb ) Genentech /Roche: Onartuzumab /OA - 5D (anti - Met mAb ) Aveo : Ficlatuzumab /AV - 299 (anti - HGF mAb ) Amgen : AMG 337 (MET TKI) Eli Lilly: LY - 2875358 (anti - Met mAb ) ArQule : Tivantinib /ARQ 197 (MET TKI) Novartis / Incyte : INCB28060/INC280 (MET TKI)
| 21 |

Fibroblast growth factor (FGF) Novartis : Dovitinib /TK1285 (FGFR, VEGFR & PDGF TKI) Galectin3 Galectin Therapeutics: GM - CT - 01 & GR - MD - 02 ( galectin antagonists) B - raf GlaxoSmithKline : Tafinlar ™/ Dabrafenib (B - raf kinase inhibitor) Genentech : Zelboraf ®/ Vemurafenib (B - raf kinase inhibitor)
| 22 |

Epidermal Growth Factor Receptor (EGFR) Genentech : Tarceva ®/ Erlotinib (EGFR TKI) Bristol - Myers Squibb & Eli Lilly: Cetuximab /Erbitux (anti - EGFR mAb ) Amgen : Vectitbix / Panitumumab (anti - EGFR mAb ) Boehringer Ingelheim : Gilotrif ™/ Afatinib (EGFR/HER2 TKI )
| 23 |

HER2 Genentech : Herceptin®/ Trastuzumab (anti - HER2 mAb ) Genentech : Kadcyla ®/Ado - trastuzumab emtansine (anti - HER2 mAb linked to DM1 drug) Genentech : Perjeta ®/ Pertuzimab (anti - HER2 mAb ) GlaxoSmithKline: Tykerb / Lapatinib (dual TKI against HER2 and EGFR) CD20 Biogen Idec & Genentech: Rituxan ®/Rituximab (anti - CD20 mAb ) GlaxoSmithKline: Arzerra ™/ Ofatumumab (anti - CD20 mAb ) Genentech: Gazyva ™/ Obinutuzumab (anti - CD20 mAb )
| 24 |

P - glycoprotein Novartis : Valspodar /PSC - 833 (P - glycoprotein inhibitor). Vascular endothelial growth factor (VEGF) & Receptor (VEGFR ) Genentech /Roche: Avastin ®/ Bevacizumab (anti - VEGF mAb ) Eli Lilly: Cyramza ™ / Ramucirumab (anti - VEGFR2 mAb ) Exelixis /GlaxoSmithKline: Foretnib /XL880/GSK1363089 (MET/VEGFR2 inhibitor ) Sanofi & Regeneron : Zaltrap ®/ Ziv - Afibercept (VEGFR1/2 IgG1 Fc fusion protein)
| 25 |

Will the Elimination of Circulating Tumor - Secreted Exosomes Improve Outcomes?
| 26 |

BY THE WAY!
| 27 |

The A e thlon Hemopurifier®
| 28 |
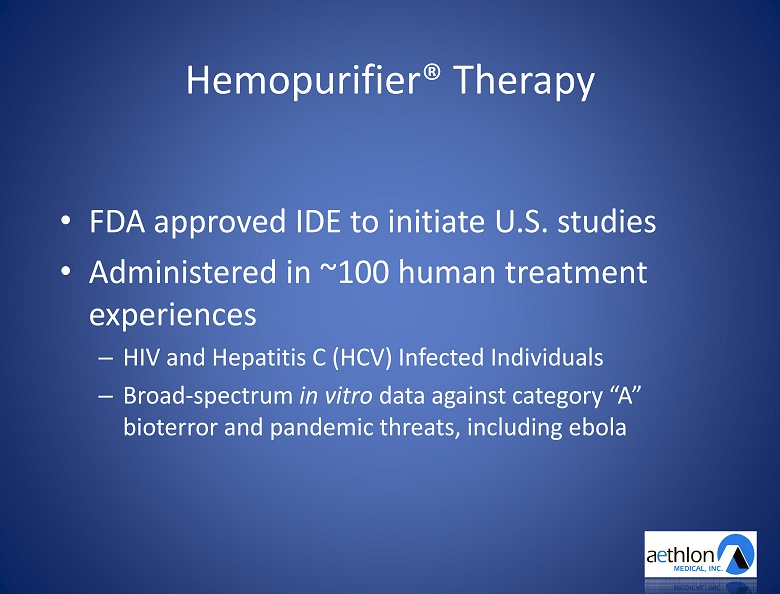
Hemopurifier ® Therapy • FDA approved IDE to initiate U.S. studies • Administered in ~100 human treatment experiences – HIV and Hepatitis C (HCV) Infected Individuals – Broad - spectrum in vitro data against category “A” bioterror and pandemic threats, including ebola
| 29 |

The A e thlon Hemopurifier® Currently Preparing Submission of Cancer IDE
| 30 |

Does the Elimination of Circulating Tumor - Secreted Exosomes Improve Outcomes?
| 31 |

We Create Targeted Therapeutic Devices to Address Infectious Disease and Cancer
| 32 |

Save Lives
| 33 |