PRESENTATION
Published on March 1, 2011
Exhibit 99.1

Jim Joyce
Chairman, CEO

Forward Looking Statements
MY PRESENTATION CONTAINS PREDICTIONS, ESTIMATES, AND OTHER FORWARD LOOKING
STATEMENTS THAT INVOLVE RISKS AND UNCERTAINTIES, INCLUDING WHETHER AND WHEN OUR
HEMOPURIFIER® AND OTHER PRODUCT OFFERINGS ARE SUCCESSFULLY DEVELOPED AND
INTRODUCED, MARKET ACCEPTANCE OF OUR HEMOPURIFIER® AND OTHER PRODUCT OFFERINGS,
REGULATORY DELAYS, MANUFACTURING DELAYS, AND OTHER RISKS DETAILED IN OUR SEC FILINGS
ACCESSIBLE ONLINE AT WWW.SEC.GOV OR WWW.AETHLONMEDICAL.COM
STATEMENTS THAT INVOLVE RISKS AND UNCERTAINTIES, INCLUDING WHETHER AND WHEN OUR
HEMOPURIFIER® AND OTHER PRODUCT OFFERINGS ARE SUCCESSFULLY DEVELOPED AND
INTRODUCED, MARKET ACCEPTANCE OF OUR HEMOPURIFIER® AND OTHER PRODUCT OFFERINGS,
REGULATORY DELAYS, MANUFACTURING DELAYS, AND OTHER RISKS DETAILED IN OUR SEC FILINGS
ACCESSIBLE ONLINE AT WWW.SEC.GOV OR WWW.AETHLONMEDICAL.COM

A revolutionary platform technology
with broad therapeutic applications
against infectious disease and cancer
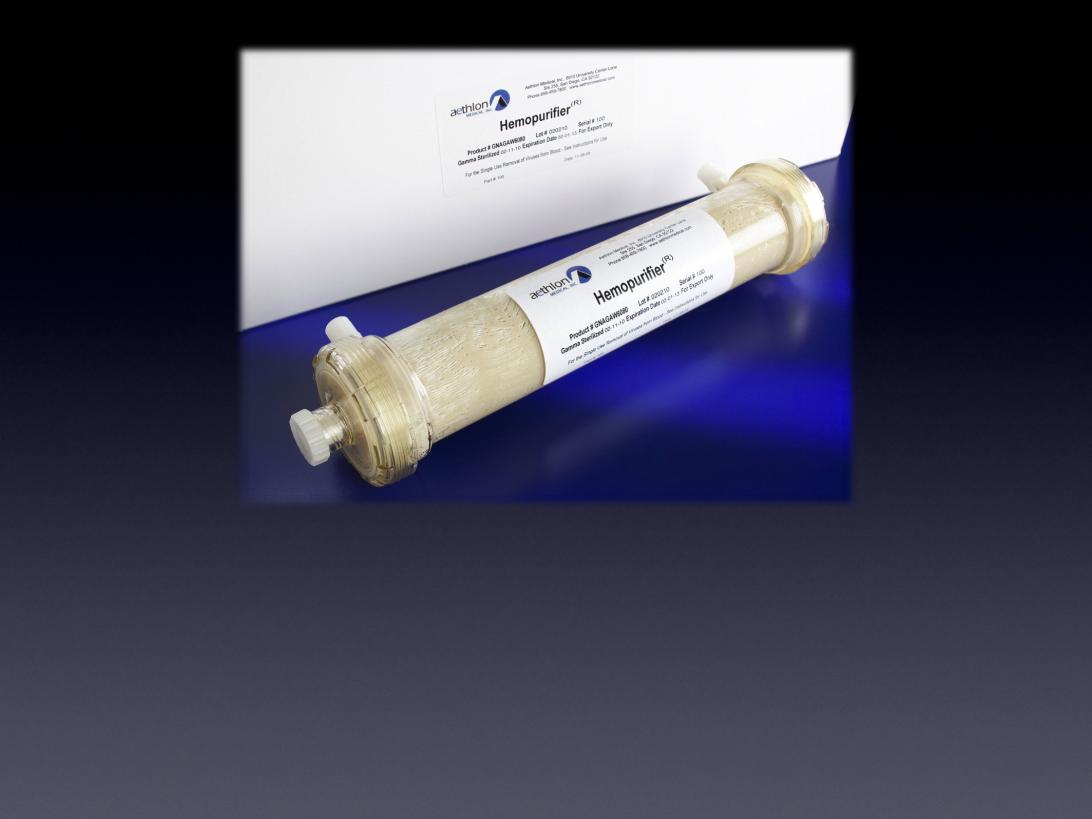
The Hemopurifier®
The first medical device to selectively remove infectious viruses
and immunosuppressive toxins from circulation
and immunosuppressive toxins from circulation

Dual Benefit of Action
Simultaneous Antiviral & Immunotherapeutic
Simultaneous Antiviral & Immunotherapeutic
• Antiviral
• Rapid real-time clearance of
infectious viral pathogens
infectious viral pathogens
• Immunotherapeutic
• Clearance of virally-shed and
cancer-secreted toxins
cancer-secreted toxins
Hepatitis-C
HIV
Cancer
Biodefense
Pandemics
Our Circle of Opportunity
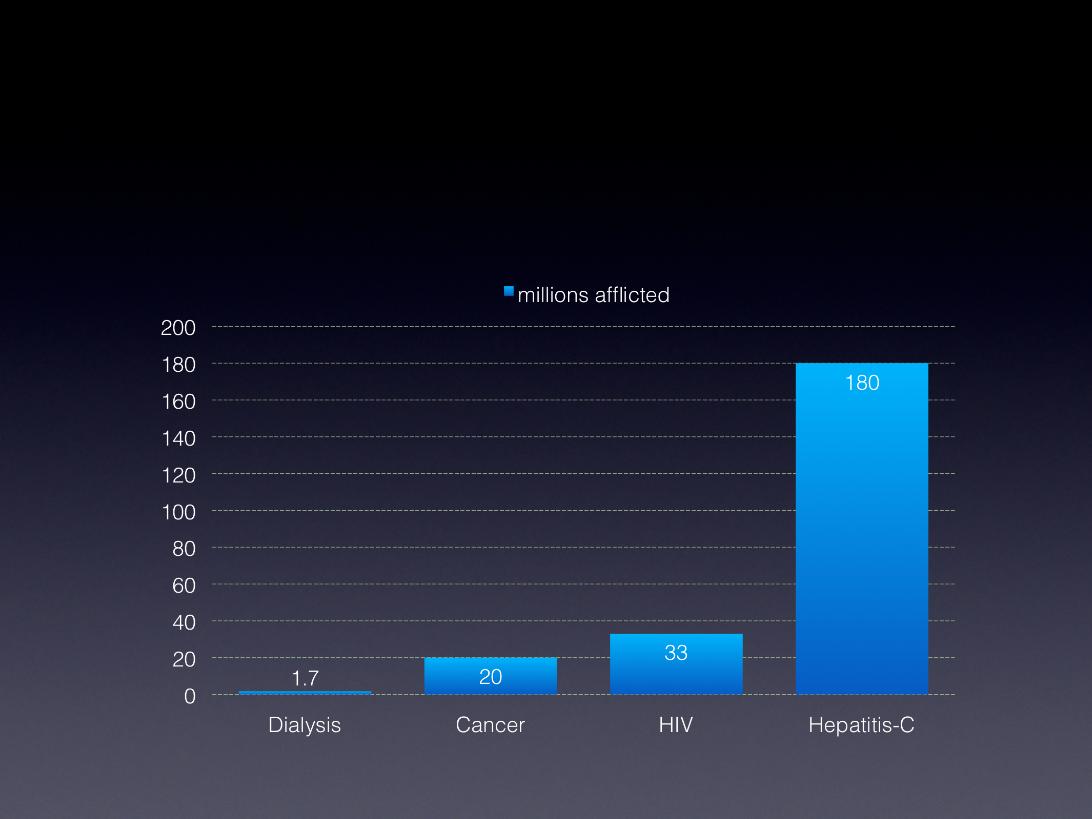
Therapeutic Filtration
The Opportunity To Transition Beyond Kidney Dialysis
The Opportunity To Transition Beyond Kidney Dialysis

|
Market Opportunity
|
Application
|
|
HCV
|
standard of care adjunct
|
|
HIV
|
drug resistant patients
|
|
Bioterror &
Pandemic Threats
|
broad-spectrum
countermeasure |
|
CANCER
|
immunotherapy / diagnostic /
biomarker |
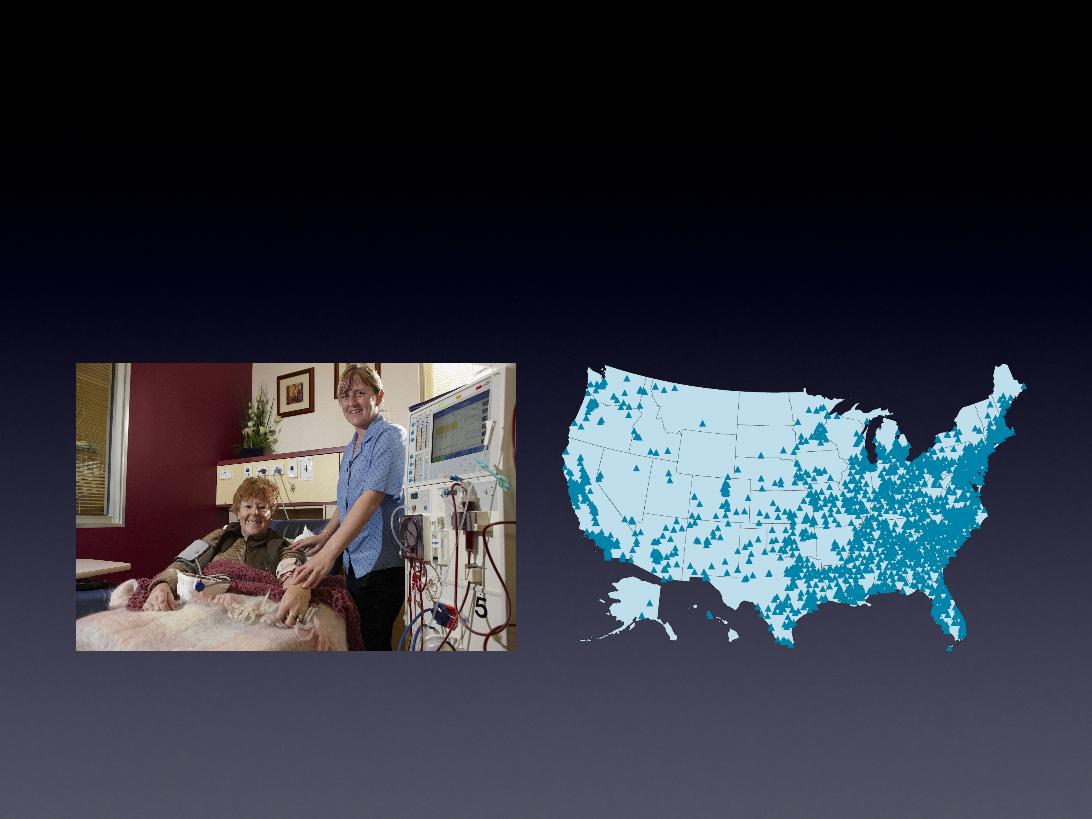
Our Hemopurifier® has the advantage of being
delivered through an established global infrastructure
of dialysis stations (90,000+ in U.S. alone)
delivered through an established global infrastructure
of dialysis stations (90,000+ in U.S. alone)
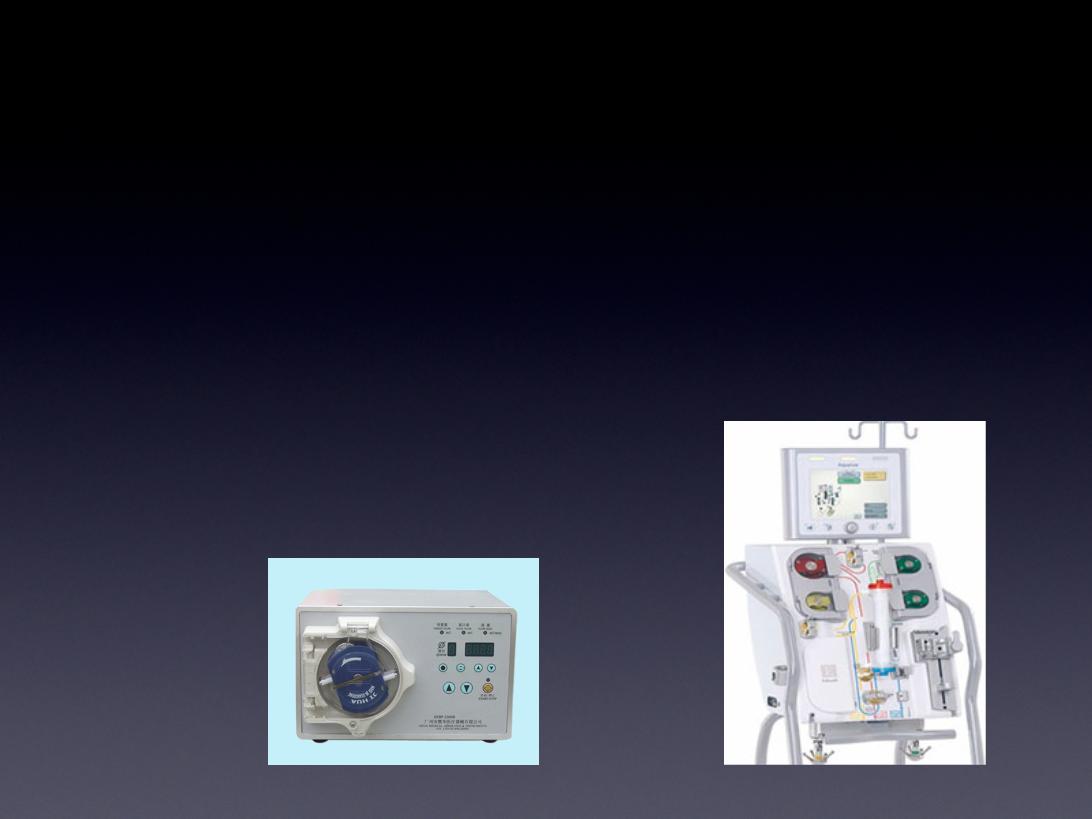
Additional Infrastructure to Deliver
Hemopurifier® Therapy Includes:
Hemopurifier® Therapy Includes:
• CRRT machines already located in hospitals
and clinics
and clinics
• Portable pump configurations

The Future
Home Infectious Disease & Cancer Therapy

Genesis of our Hemopurifier®
A 2004 patent submission entitled “A Method
for Removal of Viruses from Blood by Lectin
Affinity Hemodialysis” which issued in 2007
for Removal of Viruses from Blood by Lectin
Affinity Hemodialysis” which issued in 2007

• Broadened our intellectual property portfolio
• Completed 11 pre-clinical programs that have validated broad-spectrum effectiveness in
capturing infectious viral pathogens
capturing infectious viral pathogens
• Completed 3 separate human safety studies conducted at the Apollo, Fortis, and Sigma New
Life hospitals in India. (68 treatments)
Life hospitals in India. (68 treatments)
• Demonstrated substantial viral load reductions in HIV and HCV infected patients in the
absence of drug therapy
absence of drug therapy
• Initiated HCV adjunct clinical study designed to accelerate benefit of SOC drug therapy
• Submitted an IDE to the FDA to initiate human studies in the United States
• Established GMP manufacturing and have signed LOI to expand capabilities
• Completed 5 pre-clinical programs to validate capture of cancer-secreted exosomes
Since 2004

What Are Exosomes?

Exosomes Are Secreted By:
• All solid-form tumors
• Lymphomas
• Leukemias

Science Publications
Indicate That Exosomes
Indicate That Exosomes
• Induce Apoptosis
• Disrupt t-cell signaling
• Inhibit cytokine production
• Angiogenesis
• Metastasis
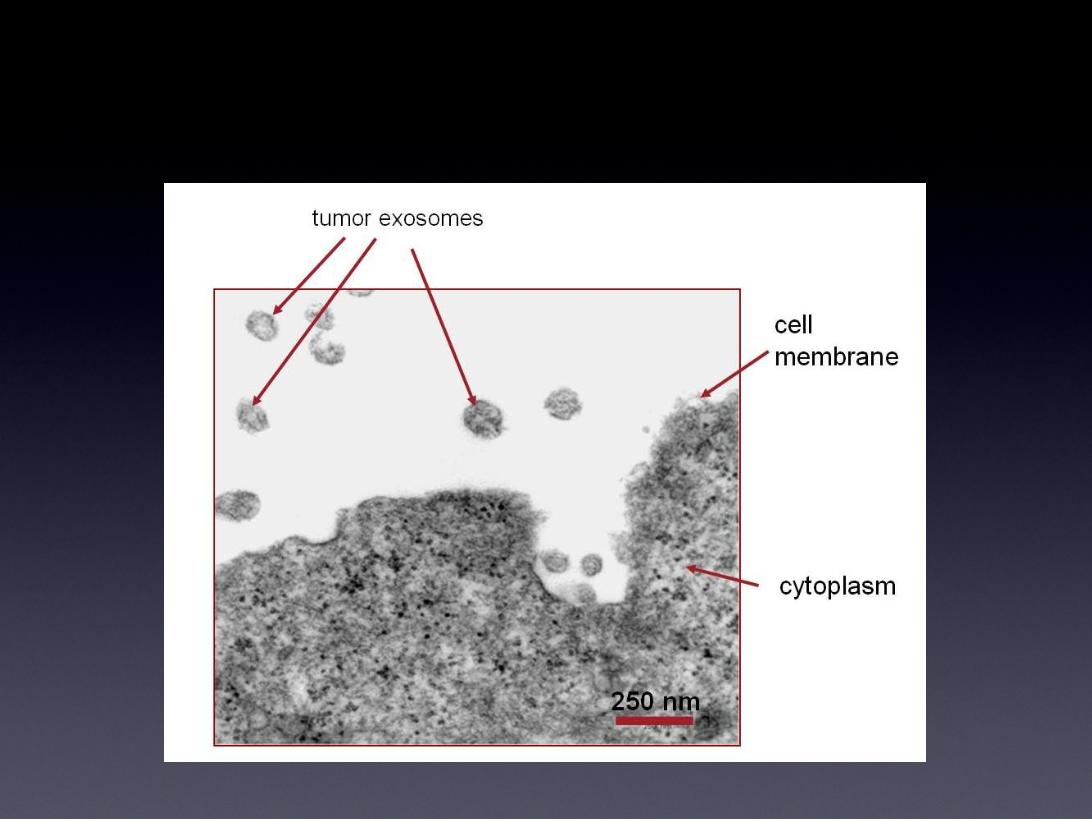
Tumor Secreted Exosomes

Pre-Clinical Hemopurifier®
Cancer Validations
Cancer Validations
• Ovarian (2008)
• Breast (2010)
• Colorectal (2010)
• Lymphoma (2010)
• Melanoma (2010)
• Additional Studies (2011)

Our Exosome
Therapeutic Opportunity
Therapeutic Opportunity
• The Hemopurifier® fills a previously unmet medical need in
cancer care
cancer care
• Provides mechanism to preserve immune function
• Serves as an adjunct to improve benefit of established and
candidate cancer therapies
candidate cancer therapies

600
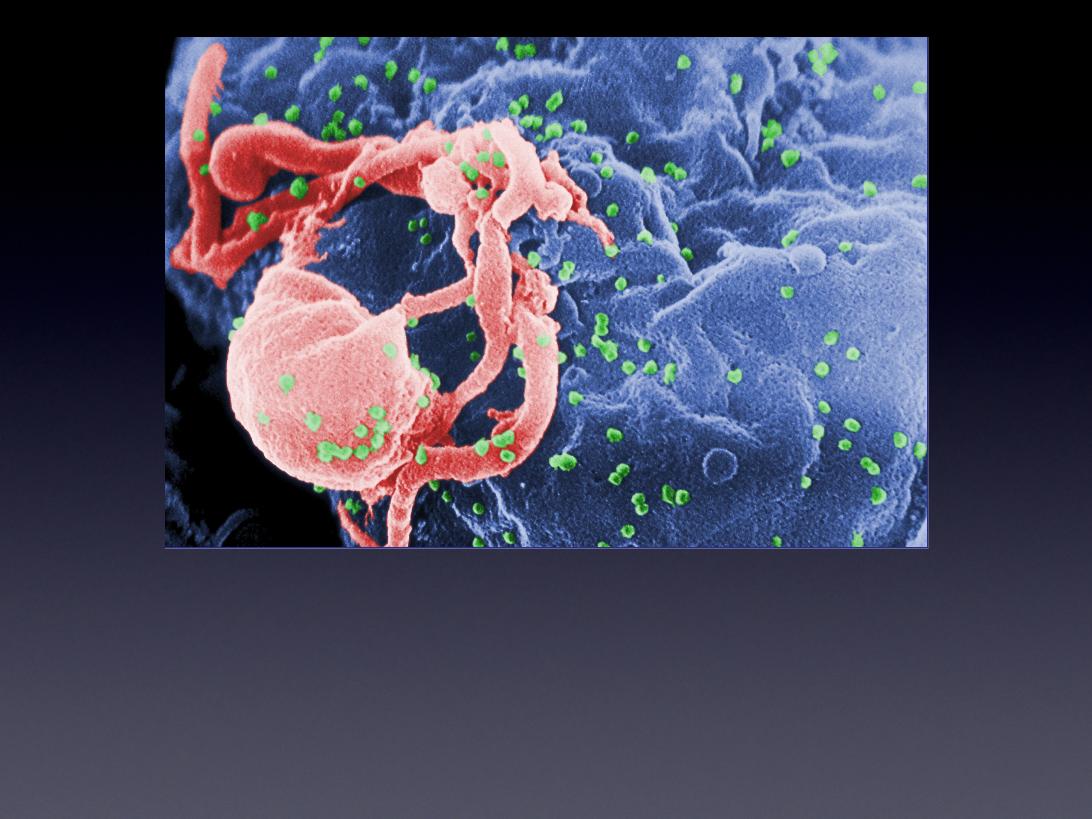
Human Immunodeficiency Virus (HIV)
A Solution to Drug Resistance
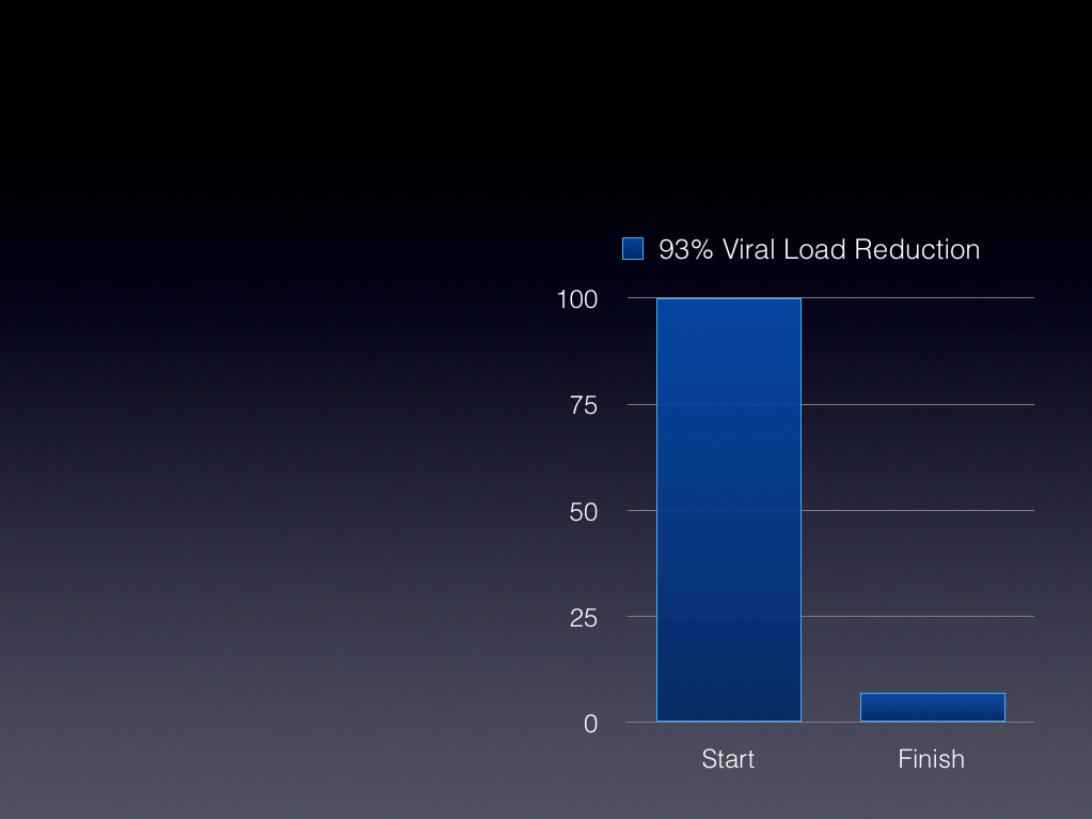
HIV-AIDS Study
• Infected dialysis patient
• 12 treatments / 30 days
• 4 hrs per treatment
• Performed in absence of drug
therapy
therapy
• Average per treatment viral load
reduction of 54%
reduction of 54%
• Improved CD4 t-cell ratios

Bioterrorism &
Pandemic Threats
Pandemic Threats

Why Return to the Biodefense
& Pandemic Threat Space?
& Pandemic Threat Space?
• HHS is committed to increasing countermeasure support
• Focal shift from one-drug-one-bug to broad-spectrum therapies
• Countermeasures with commercial market applications now ok
• FDA approval not required for stockpile consideration
• Non-dilutive funding available in amounts not limited by our
market value
market value

U.S. Department of Health and Human Services
BARDA Industry Day Presentation
Washington, DC
January 12, 2011
BARDA Industry Day Presentation
Washington, DC
January 12, 2011
James A. Joyce
Chairman, CEO

A Broad-Spectrum Antiviral Platform Technology

The New BARDA & PHEMCE
Strategic Objectives
Strategic Objectives
• To identify and support the development of innovative broad-spectrum:
• Countermeasures
• Technologies
• Platforms
• Strategies that address traditional, enhanced, emerging, and advanced
threats
threats

|
Virus
|
Collaborator
|
|
Ebola
|
USAMRIID/CDC
|
|
Dengue
|
NIV/WHO
|
|
Lassa
|
SFBR
|
|
West Nile
|
Battelle
|
|
H5N1 Avian
|
Battelle
|
|
1918-r Spanish Flu
|
Battelle
|
|
2009 H1N1 Swine
|
Battelle
|
|
Monkey Pox
|
Battelle
|
In Vitro Confirmations Against
Bioterror and Pandemic Threats
The Hemopurifier® represents the most advanced
broad-spectrum countermeasure against bioterror and
emerging pandemic threats
broad-spectrum countermeasure against bioterror and
emerging pandemic threats

"The Aethlon Hemopurifier® is the
only strategy to address the
breadth of pathogens that could be
weaponized as agents of
bioterrorism."
only strategy to address the
breadth of pathogens that could be
weaponized as agents of
bioterrorism."
Ken Alibek
Director of USSR Bioweapon Program
Author of "BIOHAZARD"

Current Activities
• Active CRADA with USAMRIID
• Defining program opportunities with BARDA
• Awaiting a BAA from DTRA
• Preparing response to BAA (released on 2/8/11) from DARPA
entitled “Dialysis Like Therapeutics”
entitled “Dialysis Like Therapeutics”
Hepatitis-C Virus (HCV)
Our #1 Priority

Why HCV?
• 180 million infected
• Low response rate to peg-interferon/ribavirin standard of care
(SOC) drug therapy
(SOC) drug therapy
• Valuations awarded to organizations with promising adjunct
data (Telaprevir from Vertex:VRTX $8.9 bn)
data (Telaprevir from Vertex:VRTX $8.9 bn)
• Clinical validation that viral filtration improves HCV cure rates

Advantages of Therapeutic Filtration
as an Adjunct to SOC
as an Adjunct to SOC
• Improves viral clearance without adding drug toxicity
• Improves viral clearance without introducing new drug
interaction risks
interaction risks
• Provides a mechanism to address all genotypes of HCV
• A device has an enduring opportunity to improve benefit of
current and future iterations of SOC drug therapy
current and future iterations of SOC drug therapy

Two therapeutic filtration strategies have
been tested in HCV infected patients
been tested in HCV infected patients
• The VRAD, a market approved device from Asahi
Kasei Kuraray Medical CO. (Japan)
Kasei Kuraray Medical CO. (Japan)
• The Hemopurifier®, a clinical stage device from
Aethlon Medical, Inc. (United States)
Aethlon Medical, Inc. (United States)
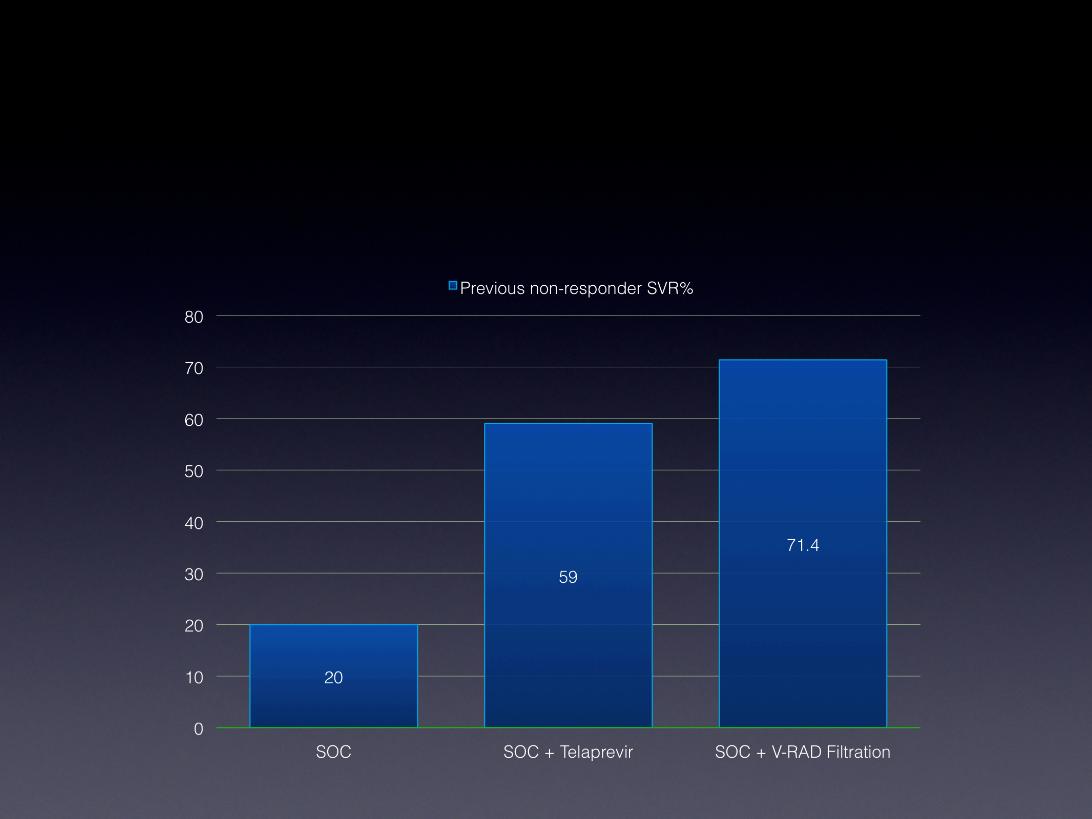
The Treatment of HCV Genotype-
1 Non-Responders
1 Non-Responders
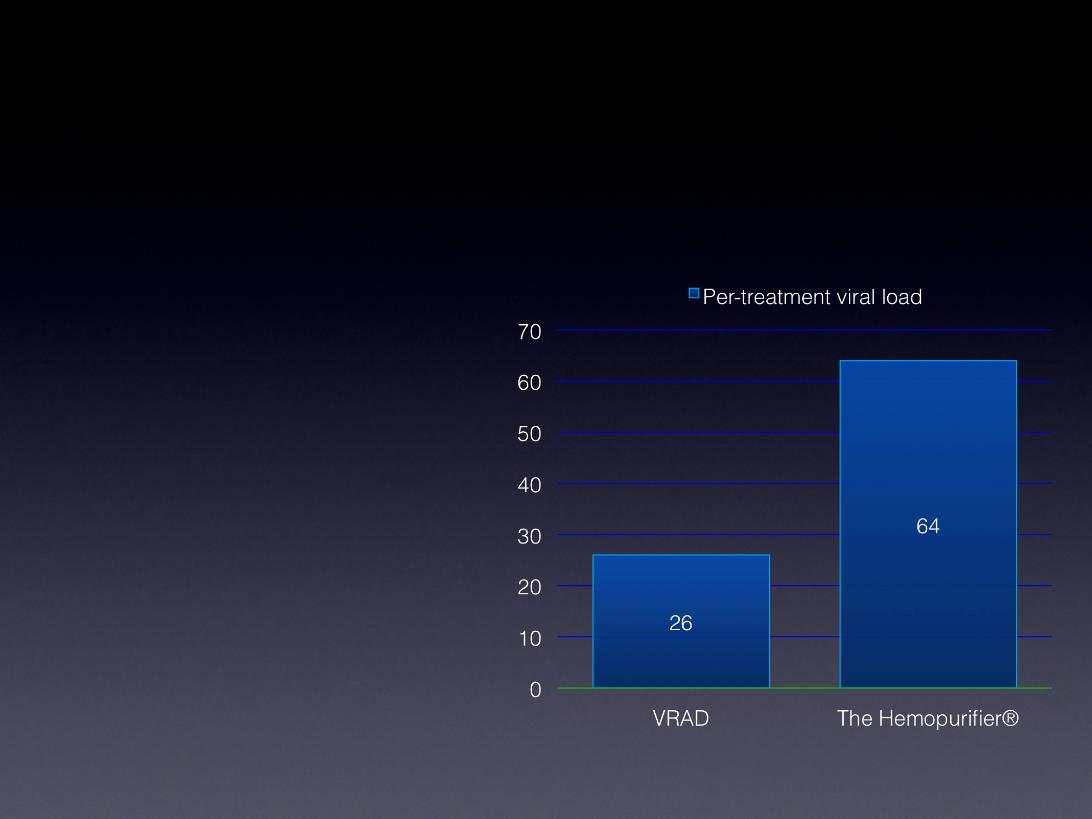
The Hemopurifier® vs. VRAD
Viral Depletion Analysis
Viral Depletion Analysis
• Average VRAD treatment period
of 3 hrs 14 min with benefit of
SOC drug therapy (n=72
treatments)
of 3 hrs 14 min with benefit of
SOC drug therapy (n=72
treatments)
• Average Hemopurifier® treatment
period of 4 hrs in absence of SOC
drug therapy benefit (n=24
treatments)
period of 4 hrs in absence of SOC
drug therapy benefit (n=24
treatments)

The Hemopurifier® vs. VRAD
• In addition to improving viral clearance
• The Hemopurifier® provides a selective capture mechanism which allows
for greater safety and optimization of treatment outcomes
for greater safety and optimization of treatment outcomes
• The Hemopurifier® removes immunosuppressive proteins shed by HCV
that cannot be addressed by VRAD
that cannot be addressed by VRAD
• The Hemopurifier® is a sealed single-use disposable cartridge vs.
multiple cartridge and pump set-required by VRAD
multiple cartridge and pump set-required by VRAD

The Hemopurifier® + SOC?
• Initiated clinical study at the Medanta Medicity Institute
• Up to 30 patients / up to 6 treatments in first 3 days of SOC
• Early clinical endpoints include:
• Immediate Virologic Response (IVR)
• Rapid Virologic Response (RVR)
• Early Virologic Response (EVR)
• Commercialization triggered upon positive outcomes

Our foundation to drive
shareholder value in 2011
shareholder value in 2011
• Positive HCV data
• Transition from R&D to revenue generation
• Commercialization
• Contract-grant income
• FDA approval to initiate U.S. clinical programs
• New cancer research data
• Strategic relationships (infectious disease and cancer)

A revolutionary platform technology
with broad therapeutic applications
against infectious disease and cancer
with broad therapeutic applications
against infectious disease and cancer

San Diego, CA. 92122
www.AethlonMedical.com

Presenter Information
Jim Joyce
Chairman, CEO
Aethlon Medical, Inc.
(858) 459-7800 x301
jj@aethlonmedical.com