INVESTOR PRESENTATION MATERIALS
Published on January 12, 2011
Exhibit 99.1

U.S. Department of Health and Human Services
BARDA Industry Day Presentation
Washington, DC
January 12, 20100
BARDA Industry Day Presentation
Washington, DC
January 12, 20100
James A. Joyce
Chairman, CEO

Forward Looking Statements
MY PRESENTATION CONTAINS PREDICTIONS, ESTIMATES, AND OTHER FORWARD LOOKING STATEMENTS THAT INVOLVE RISKS AND UNCERTAINTIES,
INCLUDING WHETHER AND WHEN OUR HEMOPURIFIER® AND OTHER PRODUCT OFFERINGS ARE SUCCESSFULLY DEVELOPED AND INTRODUCED,
MARKET ACCEPTANCE OF OUR HEMOPURIFIER® AND OTHER PRODUCT OFFERINGS, REGULATORY DELAYS, MANUFACTURING DELAYS, AND OTHER
RISKS DETAILED IN OUR SEC FILINGS ACCESSIBLE ONLINE AT WWW.SEC.GOV OR WWW.AETHLONMEDICAL.COM
INCLUDING WHETHER AND WHEN OUR HEMOPURIFIER® AND OTHER PRODUCT OFFERINGS ARE SUCCESSFULLY DEVELOPED AND INTRODUCED,
MARKET ACCEPTANCE OF OUR HEMOPURIFIER® AND OTHER PRODUCT OFFERINGS, REGULATORY DELAYS, MANUFACTURING DELAYS, AND OTHER
RISKS DETAILED IN OUR SEC FILINGS ACCESSIBLE ONLINE AT WWW.SEC.GOV OR WWW.AETHLONMEDICAL.COM

A Broad-Spectrum Antiviral Platform Technology

The BARDA Strategic Objectives
• To identify and support the development of innovative
broad-spectrum:
broad-spectrum:
• Countermeasures
• Technologies
• Platforms
• Strategies that address traditional, enhanced, emerging,
and advanced threats
and advanced threats
• Adjunct or adjuvant therapies that improve
countermeasure performance
countermeasure performance
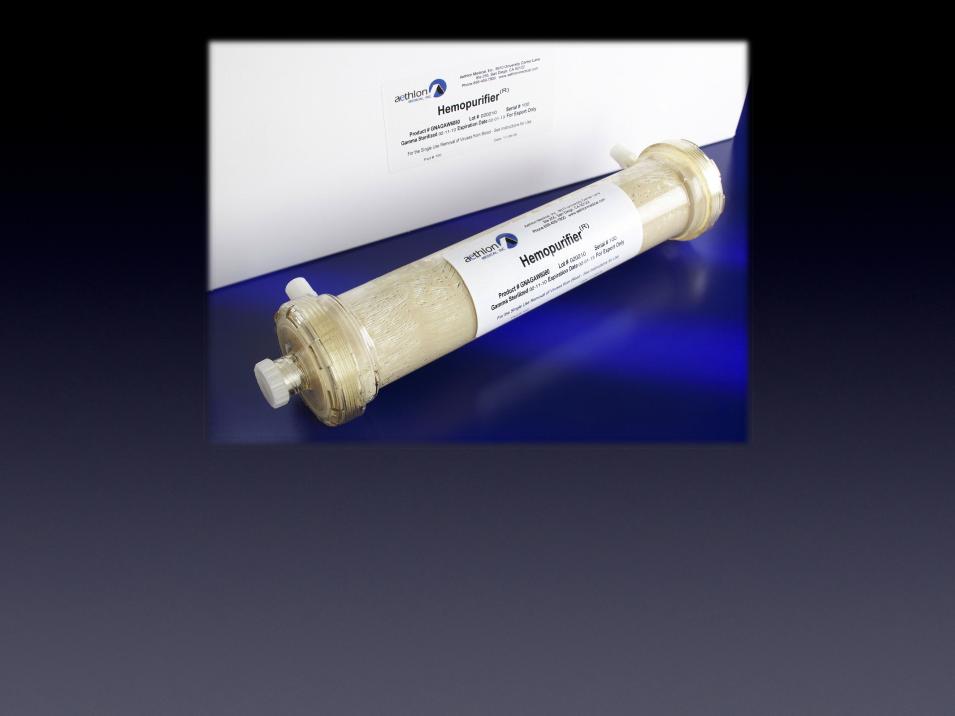
The Hemopurifier®
The first medical device to selectively remove infectious
viruses and immunosuppressive toxins from circulation
viruses and immunosuppressive toxins from circulation
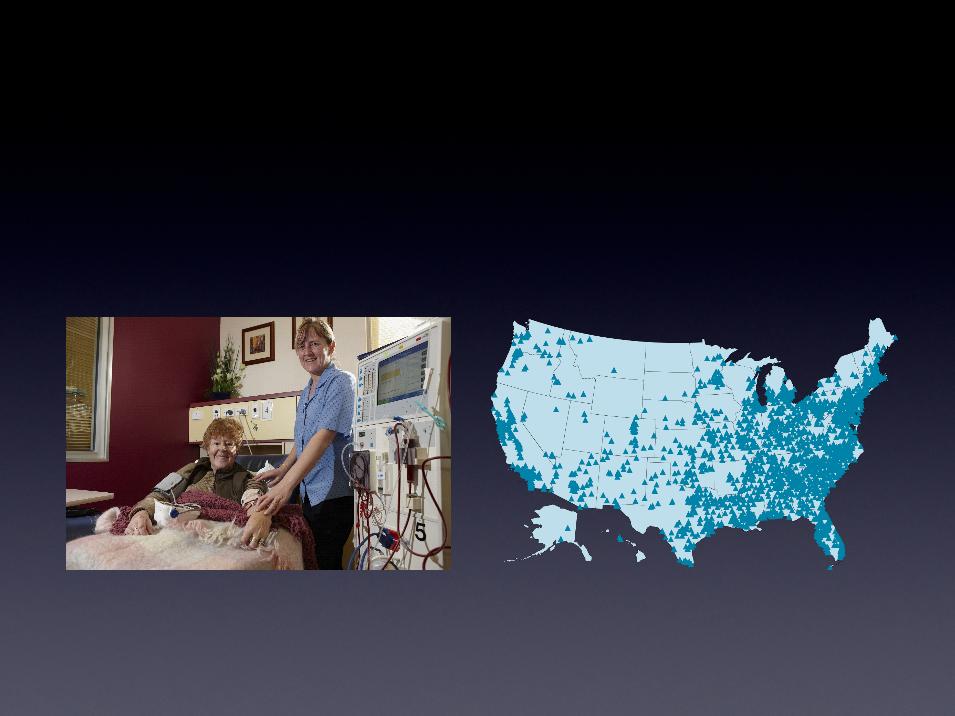
Leverages the established infrastructure
of 90,000+ U.S. based dialysis stations
of 90,000+ U.S. based dialysis stations
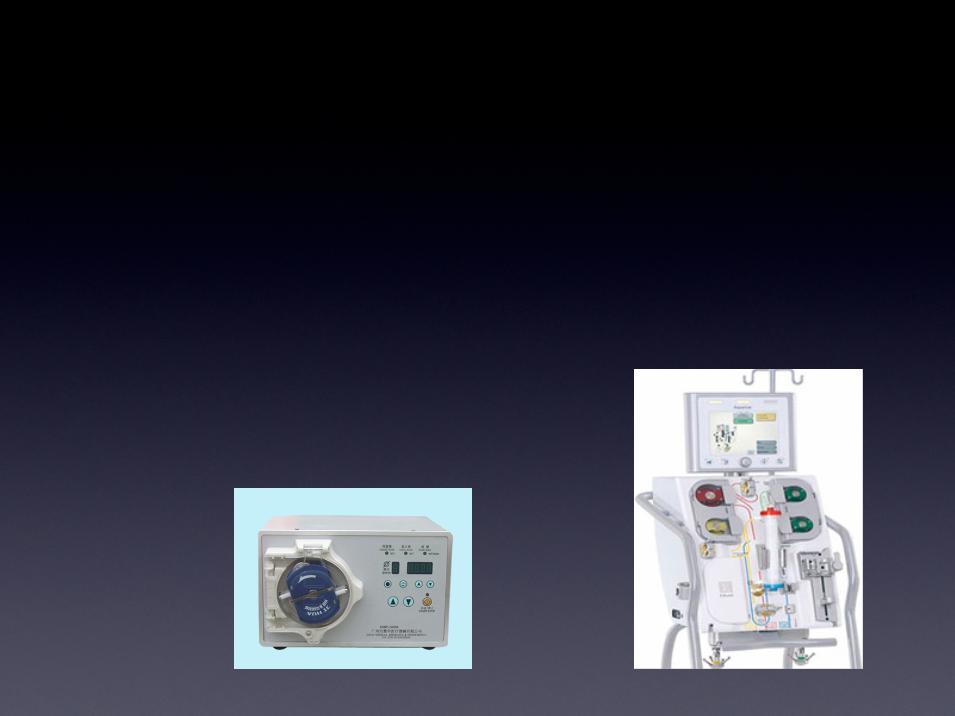
Additional Infrastructure to Deliver
Hemopurifier® Therapy
Hemopurifier® Therapy
• CRRT machines already located in hospitals
and clinics throughout the U.S.
and clinics throughout the U.S.
• Portable pump configurations
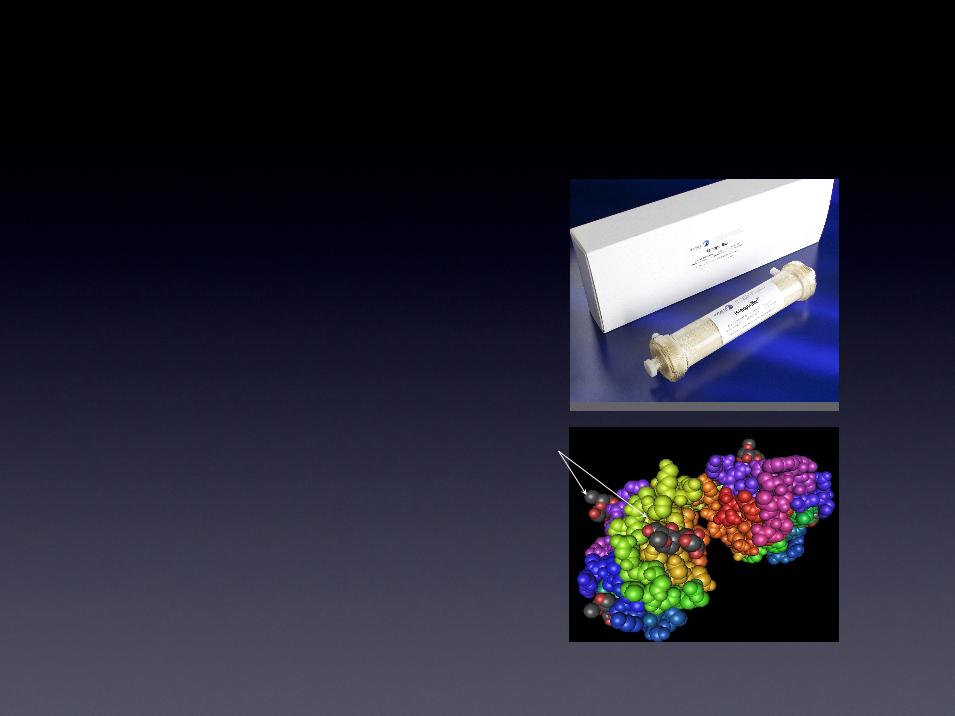
Mechanism of Action
Mannose
1. Separation of particles below
250nm from the circulatory
system
250nm from the circulatory
system
2. Immobilized lectin affinity agents
then selectively bind to unique
high mannose structures resident
on viral glycoproteins that coat
viral pathogens
then selectively bind to unique
high mannose structures resident
on viral glycoproteins that coat
viral pathogens
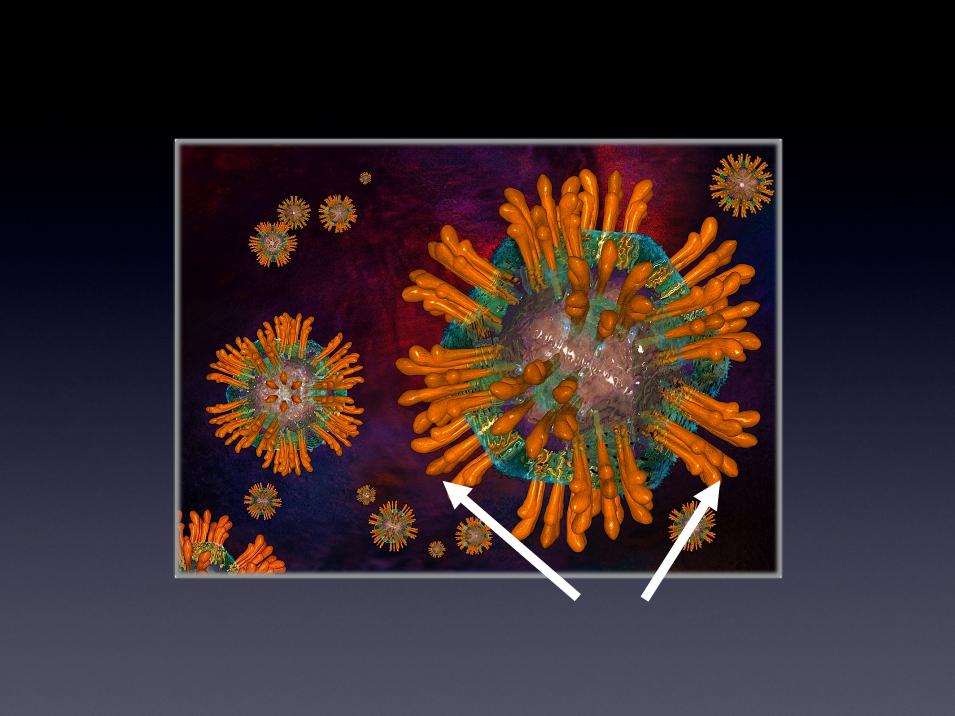
Lectin Binding Sites

Dual Benefit of Action
Antiviral & Immunotherapeutic
Antiviral & Immunotherapeutic
• Antiviral
• Rapid real-time clearance of
infectious viral pathogens
infectious viral pathogens
• Immunotherapeutic
• Fulfills unmet medical need of
clearing virally-shed
immunosuppressive
glycoproteins
clearing virally-shed
immunosuppressive
glycoproteins

• Regulatory Path: Device vs. Drug
• 70 human treatment experiences
• IDE on file with FDA
• GMP manufacturing already established
• Substantial viral load reductions in human HIV and HCV studies
• Follow-on HCV studies initiated
• Proven broad-spectrum capabilities against bioterror and pandemic
threats
threats
Hemopurifier®
Selected Quick Facts

|
Virus
|
Collaborator
|
|
Ebola
|
USAMRIID/CDC
|
|
Dengue
|
NIV/WHO
|
|
Lassa
|
SFBR
|
|
West Nile
|
Battelle
|
|
H5N1 Avian
|
Battelle
|
|
1918-r Spanish Flu
|
Battelle
|
|
2009 H1N1 Swine
|
Battelle
|
|
Monkey Pox
|
Battelle
|
In Vitro Confirmations Against
Bioterror and Pandemic Threats

The Hemopurifier® improves public
health emergency preparedness against:
health emergency preparedness against:
• Traditional Threats
• Enhanced Agents
• Emerging Pathogens
• Advanced Agents

"The Aethlon Hemopurifier® is the
only strategy to address the
breadth of pathogens that could
be weaponized as agents of
bioterrorism."
only strategy to address the
breadth of pathogens that could
be weaponized as agents of
bioterrorism."
Ken Alibek
First Deputy Director of Biopreparat

Traditional Threats
Known naturally occurring threats such as
Smallpox, Ebola, Marburg, Lassa
Known naturally occurring threats such as
Smallpox, Ebola, Marburg, Lassa
• The Hemopurifier® serves as an adjunct to improve the
benefit of established and candidate treatment strategies
benefit of established and candidate treatment strategies
• The Hemopurifier® provides a first-line countermeasure
strategy against threats not addressed by drug or vaccines
strategy against threats not addressed by drug or vaccines

Enhanced Agents
Traditional threats that have been genetically modified to
enhance virulence or circumvent drug and vaccine therapies
Traditional threats that have been genetically modified to
enhance virulence or circumvent drug and vaccine therapies
• The Hemopurifier® serves as a first-line countermeasure
against enhanced agents
against enhanced agents
• Provides adjunct strategy to strengthen benefit of
therapies proven safe and effective against similar
pathogen threats
therapies proven safe and effective against similar
pathogen threats
• Assists in characterization of enhanced agents

Emerging Pathogens
Previously unrecognized pathogens that occur naturally such as
SARS or future strains of pandemic influenza
Previously unrecognized pathogens that occur naturally such as
SARS or future strains of pandemic influenza
• The Hemopurifier® is a candidate first-line
countermeasure against emerging pathogens
countermeasure against emerging pathogens
• Provides adjunct strategy to strengthen benefit of
therapies proven safe and effective against similar
pathogen threats
therapies proven safe and effective against similar
pathogen threats
• Assists in early characterization of emerging
pathogen threats
pathogen threats

Advanced Agents
Novel pathogen artificially engineered to bypass traditional drug
and vaccine countermeasures or enhance disease severity
Novel pathogen artificially engineered to bypass traditional drug
and vaccine countermeasures or enhance disease severity
• The Hemopurifier® represents the sole first-line
treatment strategy against advanced agents
treatment strategy against advanced agents
• Assists in early characterization of advanced agents

The Hemopurifier® also provides a therapeutic
mechanism to address at-risk populations for whom drug
and vaccine therapies may be contraindicated
mechanism to address at-risk populations for whom drug
and vaccine therapies may be contraindicated
• Immunocompromised
• Children
• Pregnant women
• Senior Citizens

Stockpile Implications
• > 4 year shelf life
• Refrigeration not required
• Therapeutic demand in HCV, HIV, and cancer provides
replenishing FIFO stockpile
replenishing FIFO stockpile

Selected Examples of Therapeutic Filtration
• Kidney dialysis
• Hepatitis-C Virus
• Marburg Plasmapheresis (1990)
• Anthrax Toxin Plasmapheresis (2001)
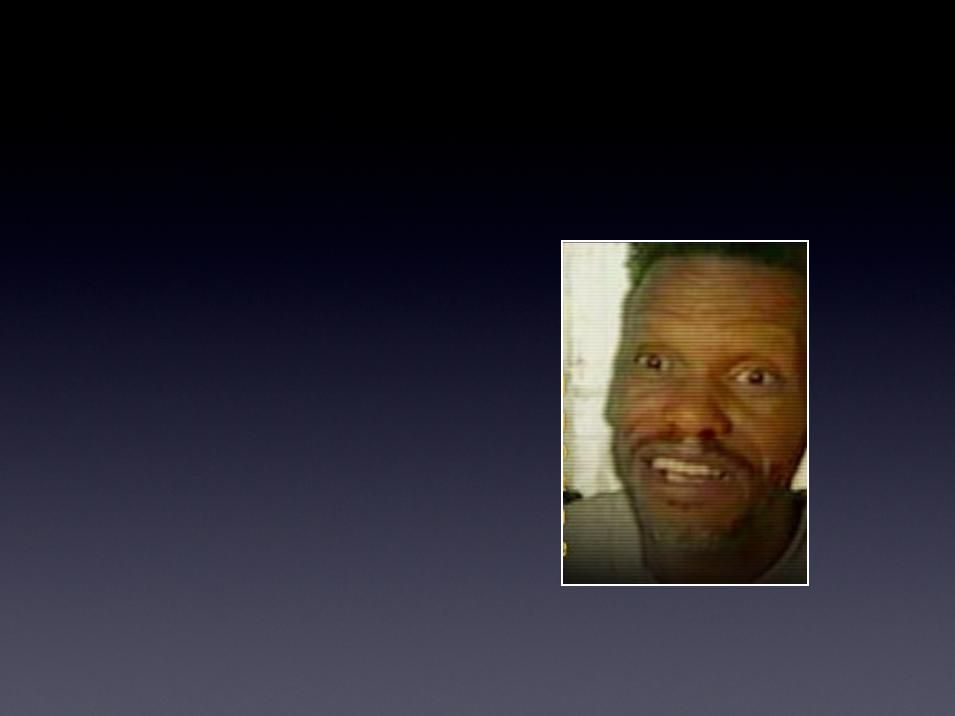
2001 Anthrax Survivor
"It's hard to say what saved me, but one
of the things was plasmapheresis.
Honestly, without it, I would be dead."
of the things was plasmapheresis.
Honestly, without it, I would be dead."
Leroy Richmond
Anthrax infected postal worker

An Adaptable Platform Technology
• The Hemopurifier® provides a platform for multi-use product
development and manufacture
development and manufacture
• Interchanging affinity agents within our core Hemopurifier®
cartridge expands targets beyond viral threats
cartridge expands targets beyond viral threats
• Bacterial threats such as Anthrax could be addressed through
immobilization of corresponding anti-toxin antibodies or binding
agents
immobilization of corresponding anti-toxin antibodies or binding
agents
• Radiological threats could be addressed through
immobilization of corresponding chelating agents(s)
immobilization of corresponding chelating agents(s)

Conclusions
• The Hemopurifier® fulfills BARDA's strategy objectives
• It is an innovative broad-spectrum medical countermeasure
• Offers a strategy to address traditional, enhanced, emerging,
and advanced threats
and advanced threats
• Serves as an adjunct to enhance the benefit of drug and
vaccine countermeasures
vaccine countermeasures
Conclusion
The Aethlon Hemopurifier® represents the most advanced broad-
spectrum countermeasure against bioterror and emerging
pandemic threats
spectrum countermeasure against bioterror and emerging
pandemic threats

8910 University Center Lane, Suite 660
San Diego, CA. 92122
www.AethlonMedical.com

Presenter Information
Jim Joyce
Chairman, CEO
Aethlon Medical, Inc.
(858) 459-7800 x301
jj@aethlonmedical.com