424B3: Prospectus filed pursuant to Rule 424(b)(3)
Published on April 15, 2015
Filed pursuant to Rule 424(b)(3)
Registration No. 333-201334
PROSPECTUS SUPPLEMENT NO. 6
(to prospectus dated January 28, 2015)
Aethlon Medical, Inc.
495,000 Shares of Common Stock
This prospectus supplement relates to the prospectus dated January 28, 2015 relating to the following common stock that may be sold from time to time by the selling stockholders identified in the prospectus:
| · | 220,000 shares of common stock; and |
| · | 275,000 shares of common stock underlying common stock purchase warrants at an exercise price of $15.00 per share. |
All of the common stock covered by the prospectus is being sold by the selling stockholders for their own account. We will not receive any proceeds from the sale of these shares other than proceeds, if any, from the exercise of warrants to purchase shares of our common stock. If all of the warrants are exercised for cash, we will receive a total of $4,125,000 in gross proceeds, which we expect to use for general corporate purposes. We cannot assure you that any warrants will be exercised for cash. The selling stockholders may offer and sell the shares covered by the prospectus at prevailing prices quoted on the OTCQB Marketplace or at privately negotiated prices. The selling stockholders may sell the shares directly or through underwriters, brokers or dealers. The selling stockholders will bear any applicable sales commissions, transfer taxes and similar expenses. We will pay all other expenses incident to the registration of the shares. See “Plan of Distribution” on page 28 of the prospectus for more information on this topic. The selling stockholders originally purchased the common stock and warrants from us on December 2, 2014, for an aggregate price of $3,300,000. The prospectus covers the sale of those securities by the selling stockholders.
We are filing this prospectus supplement to supplement and amend the information previously included in the prospectus with the information contained in our Current Report on Form 8-K filed with the Securities and Exchange Commission on April 15, 2015, regarding a clinical trial agreement with the University of California, Irvine. Accordingly, we have attached our Current Report on Form 8-K to this prospectus supplement. You should read this prospectus supplement together with the prospectus and the prospectus supplements filed on February 10, 2015, March 16, 2015, April 7, 2015, April 10, 2015 and April 14, 2015, which are to be delivered with this prospectus supplement.
Our common stock is quoted on the OTCQB Marketplace under the symbol “AEMD.” On April 14, 2015, the last quoted sale price of our common stock as reported on the OTCQB Marketplace was $12.00 per share.
Investing in our securities involves significant risks, including those set forth in the “Risk Factors” section of the prospectus beginning at page 4.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THE PROSPECTUS OR THIS OR ANY OTHER PROSPECTUS SUPPLEMENT IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus supplement is April 15, 2015.
| 1 |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): April 15, 2015 (April 9, 2015)
AETHLON MEDICAL, INC.
(Exact name of registrant as specified in its charter)
|
Nevada (State or other jurisdiction of incorporation) |
000-21846 (Commission File Number) |
13-3632859 (IRS Employer Identification Number) |
|
9635 Granite Ridge Drive, Suite 100 San Diego, California (Address of principal executive offices) |
92123 (Zip Code) |
Registrant’s telephone number, including area code: (858) 459-7800
Not applicable
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2 below):
| o | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) | |
| o | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) | |
| o | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) | |
| o | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| 2 |
FORWARD-LOOKING STATEMENTS
This Form 8-K and other reports filed by Registrant from time to time with the Securities and Exchange Commission (collectively, the "Filings") contain or may contain forward-looking statements and information that are based upon beliefs of, and information currently available to, Registrant's management as well as estimates and assumptions made by Registrant's management. When used in the Filings the words "anticipate,” "believe," "estimate," "expect," "future," "intend," "plan" or the negative of these terms and similar expressions as they relate to Registrant or Registrant's management identify forward-looking statements. Such statements reflect the current view of Registrant with respect to future events and are subject to risks, uncertainties, assumptions and other factors relating to Registrant's industry, Registrant's operations and results of operations and any businesses that may be acquired by Registrant. Should one or more of these risks or uncertainties materialize, or should the underlying assumptions prove incorrect, actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned.
Although Registrant believes that the expectations reflected in the forward-looking statements are reasonable, Registrant cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, Registrant does not intend to update any of the forward-looking statements to conform these statements to actual results.
ITEM 1.01 ENTRY INTO A MATERIAL DEFINITIVE AGREEMENT.
On April 9, 2015, Aethlon Medical, Inc. (“we”) entered into an investigator-initiated clinical trial agreement (the “Agreement”) with the University of California, Irvine (“UCI”). Under the Agreement, UCI will conduct a five-year clinical study protocol entitled “Plasma Exosome Concentration in Cancer Patients Undergoing Treatment” (the “Protocol”). The Protocol will seek to enroll five (5) individuals in each of nine defined tumor types for a total study population of up to forty-five (45) subjects (the “Clinical Trial”). The tumor types include the following forms of cancer; Breast adenocarcinoma, Colorectal, Gastric & Gastroesophageal, Pancreatic, Cholangiocarcinoma, Lung (NSCLC), Head & Neck (SCC), Melanoma and Ovarian adenocarcinoma. The principal investigator of the study is Edward Nelson, M.D.
The budget for the Protocol provides for (i) $19,032 in startup charges; (ii) $8,039 in Protocol related variable pass-through charges; and (iii) per subject visit charges of $3,359 per subject, for a total subject visit charge of $151,155 for forty-five (45) subjects. These costs will be borne by us. UCI may disseminate the results of the Clinical Trial through presentation and/or publication, but may not disclose any of our confidential information.
The foregoing description of the Agreement, the Protocol, the Clinical Trial and the budget does not purport to be complete and is qualified in its entirety by the Clinical Trial Agreement attached hereto as Exhibit 10.1, the Protocol attached hereto as Exhibit 10.2, and the Budget attached hereto as Exhibit 10.3, each of which is incorporated herein by reference.
ITEM 8.01 OTHER EVENTS.
On April 15, 2015, the Registrant issued a press release announcing the Clinical Trial Agreement with UCI. A copy of the press release is attached hereto as Exhibit 99.1 and incorporated herein by reference.
ITEM 9.01 FINANCIAL STATEMENTS AND EXHIBITS.
| EXHIBIT NO. | DESCRIPTION |
| 10.1 | UCI Clinical Trial Agreement |
| 10.2 | Protocol for UCI Clinical Trial |
| 10.3 | Budget for UCI Clinical Trial |
| 99.1 | “Aethlon Medical Announces Cancer Clinical Trial Agreement” |
| 3 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| AETHLON MEDICAL, INC. | ||||
|
By: /s/ James B. Frakes |
||||
| James B. Frakes | ||||
| Dated: April 15, 2015 | Chief Financial Officer |
| 4 |
Exhibit 10.1
CLINICAL TRIAL AGREEMENT NO. AMI-201951
This clinical trial agreement (“Agreement”) is entered into this 24th day of March 2015, by and between The Regents of the University of California, on behalf of its Irvine campus, a California corporation with a place of business at 5171 California Avenue, Suite 150, Irvine, CA 92697-7600 (hereinafter referred to as the “Institution”) and Aethlon Medical, Inc. and its subsidiaries, a Nevada corporation with a place of business at 9635 Granite Ridge Drive, Suite 100, San Diego, CA. 92123, (hereinafter referred to as the “Sponsor"), the Institution and Sponsor each being a “party“ and hereinafter collectively referred to as the “Parties”.
WITNESSETH
WHEREAS, Sponsor is interested in supporting a clinical trial by providing financial support;
WHEREAS, the Institution acknowledges and affirms that it is familiar with and understands the regulations of the United States Food and Drug Administration governing clinical trials and that it is capable of complying with such regulations as applicable to this trial; and
WHEREAS, the Institution and Sponsor have entered into this Agreement in order to set forth the terms pursuant to which a clinical trial shall be performed by the Institution.
NOW THEREFORE, the Parties agree as follows:
| 1. | SCOPE OF WORK | |
| A. | The Institution shall exercise reasonable efforts to conduct the investigator-initiated clinical trial (“Clinical Trial”) set forth in the protocol entitled "Plasma Exosome Concentration in Cancer Patients Undergoing Treatment" ("Protocol"), in accordance with this Agreement. The Protocol, which is approved by the Sponsor and the Institution’s Institutional Review Board (“IRB”), is attached hereto as Exhibit A and incorporated by reference (along with any approved subsequent modifications). In the event of any conflict between the terms of this Agreement and the Protocol, the terms of this Agreement shall govern. |
| 5 |
| 2. | PRINCIPAL INVESTIGATOR |
The Institution's principal investigator shall be Edward Nelson, M.D. ("Principal Investigator") who shall be responsible for the direction of the Clinical Trial in accordance with applicable Institution policies. If, for any reason, (s)he is unwilling or unable to continue to serve as the Principal Investigator and a successor, acceptable to both the Institution and Sponsor, is not available, this Agreement may be terminated by either party in accordance with the provisions of Paragraph A of Section 13.
| 3. | PERIOD OF PERFORMANCE |
This Agreement shall commence on March 24, 2015 and shall continue through March 23, 2020, unless extended by mutual written agreement of the Parties or unless sooner terminated pursuant to Paragraph A of Section 13.
| 4. | RECORDKEEPING, REPORTING AND ACCESS |
| A. | The Institution shall notify Sponsor of each serious adverse event encountered in the Clinical Trial within twenty-four (24) hours of becoming aware of it. | |
| B. | The Institution shall, in a timely manner, prepare and maintain complete and accurate written records related to the Clinical Trial (“Records”). | |
| D. | The Institution will provide the Sponsor with quarterly updates concerning the progress of the Clinical Trial. Upon completion of Clinical Trial, or earlier termination of this Agreement, the Institution will provide a final written report (“Final Report”) to Sponsor describing the outcomes of the work performed. |
| 6 |
5. COSTS, PAYMENT
| A. | As consideration for Institution’s performance of the Clinical Trial under the terms of this Agreement, Sponsor agrees to pay Institution in accordance with the terms of Exhibit B, which is attached hereto and incorporated by reference. |
| B. | All costs set forth in Exhibit B shall remain firm for the duration of the Clinical Trial, unless otherwise agreed to in writing by the Parties. |
| C. | Checks shall be made payable to “The Regents of the University of California.” Checks shall reference this Agreement number and shall be mailed to the address shown in Exhibit B. |
| 6. | CONFIDENTIAL INFORMATION |
| A. | The Institution shall not disclose or use for any purpose other than the performance of the Clinical Trial any privileged records and other confidential or proprietary information disclosed to the Institution by or on behalf of Sponsor (“Confidential Information“). Confidential Information shall be marked as confidential or, if not so marked, shall be treated as confidential if a reasonable person involved in the conduct of clinical trials would reasonably believe such information was a trade secret, confidential or proprietary information of Sponsor. This obligation of non-disclosure and non-use shall not apply to any Confidential Information: |
| (1) | that is or becomes publicly available through no fault of the Institution; |
| (2) | already known to the Institution prior to receipt from Sponsor, as shown by Institution’s prior written records; |
| (3) | disclosed to the Institution by a third-party without an obligation of confidentiality; |
| (4) | developed and/or discovered independently by Institution without reference to and/or use of Confidential Information, as evidenced by written contemporaneous documentation; and |
| (5) | required to be released by any governmental entity with jurisdiction, provided that the Institution gives notice to Sponsor at least ten (10) days (if reasonably possible, and, if not, as many days as is reasonably possible) prior to making such release of Confidential Information. |
| B. | The obligations of the Institution under this Section shall survive the termination of this Agreement for a period of five (5) years. |
7. PRESENTATIONS AND PUBLICATIONS
| A. | The Institution may, consistent with scientific standards, disseminate the results of the Clinical Trial through either presentation or publication including, but not limited to, full papers, manuscripts, abstracts, poster presentations, and oral presentations (“Publications”), but will not disclose any Confidential Information without Sponsor' prior written consent. |
| B. | Institution shall submit Publications to Sponsor for review thirty (30) days prior to any publication. Sponsor may comment upon, but may not make any editorial changes to, the results and conclusions set forth in the Publications; however, if identified by Sponsor, the Institution will delete any of Sponsor's Confidential Information that may be contained therein. In the event that Sponsor determines patentable subject matter is disclosed in a Publication, it shall immediately notify Institution and, if Institution concurs, such Publication will be delayed: (a) for up to ninety (90) days to permit preparation and filing of appropriate patent application(s); (b) until a patent application thereon has been prepared and filed; or (c) until Institution and Sponsor mutually agree in writing that no patent application(s) shall be prepared or filed, whichever of (a), (b), or (c) is earlier in time. |
| 7 |
8. INTELLECTUAL PROPERTY
| A. | Title to any inventions and discoveries conceived and reduced to practice in the performance of the Clinical Trial ("Invention") shall be determined in accordance with the rules of inventorship under United States Patent law in effect at the time of the invention. Sponsor shall own each Invention made solely by one or more inventors under an obligation to assign inventions to Sponsor (“Sponsor Inventions”). Institution shall own each Invention made solely by one or more inventors under an obligation to assign inventions to Institution (“Institution Inventions”). The Parties shall jointly own each Invention jointly made by one or more inventors under an obligation to assign inventions to the Institution and one or more inventors under an obligation to assign inventions to Sponsor (“Joint Inventions”). Each party shall make all decisions concerning, and shall bear all expenses of, the patenting of, and the maintenance of patents on, each Invention that it owns and shall own the resulting applications and patents. In the case of Joint Inventions, the Parties shall enter into good faith negotiations concerning the patenting thereof and the maintenance of patents thereon. |
| B. | To the extent that Institution has the legal right to do so, Sponsor shall be given a time-limited first right to negotiate an exclusive, worldwide, royalty-bearing commercial license to all rights, title and interest which Institution may have or obtain in Joint Inventions and in Institution Inventions, with such license to include the right to make, use and sell such Inventions. | |
| C. | Institution shall promptly disclose to Sponsor in writing any Joint Inventions and Institution Inventions. Sponsor shall hold such disclosure on a confidential basis and will not disclose the information to any third party without consent of the Institution. Sponsor shall advise Institution in writing within sixty (60) days of disclosure to Sponsor whether it wishes to secure a commercial license for any such Inventions. If Sponsor elects to secure such a license, Sponsor shall assume all costs associated with securing and maintaining patent protection for such Invention(s), whether Letters Patent issue or not. Sponsor shall have ninety (90) days from the date of election to conclude a license agreement with Institution. Said license shall contain reasonable terms, and shall require diligent performance by Sponsor for the timely commercial development and early marketing of such Inventions, and include Sponsor's continuing obligation to pay patent cost. If such license agreement is not concluded in said period, Institution has no further obligations to Sponsor. If Sponsor does not elect to secure such license, rights to the Inventions disclosed hereunder shall be disposed of in accordance with Institution policies with no further obligation to Sponsor. |
| D. | Nothing contained in this Agreement shall be deemed to grant, either directly or by implication, estoppel or otherwise, any license under any patents, patent applications or other proprietary interests of any other invention, discovery or improvement of either party. |
| 9. | PUBLICITY |
Neither party shall use the other party's name in advertising nor publicity, without the prior written permission of the other party, except as required by law (and, in such case, only with ten (10) days prior notice to the other party (if reasonably possible, and, if not, as many days as is reasonably possible). If the relationship is deemed material under U.S. securities law, the parties recognize any required public disclosure shall be on a timely basis. Such prior permission shall not be unreasonably withheld. Institution, as an instrumentality of the State of California, is subject to a State requirement that the Institution make available the terms and conditions of its contracts. Sponsor acknowledges that Institution maintains a publicly accessible listing of all proposals and awards; the listing includes the name of the campus, sponsor, award amount, begin and end dates, principal investigator and co-investigator’s name, project type, award instrument, descriptive project title, indirect cost rate, account and fund number, department and academic discipline. The actual contract agreement must be released upon request, although portions of the document may be withheld when redaction meets one of the legal exemptions under the California Public Records Act. As such, the general terms and conditions of this Agreement will be released to the public upon request, although portions of the scope of work may be withheld to the extent that patentable inventions are so disclosed.
| 8 |
| 10. | APPLICABLE LAW |
This Agreement shall be governed by the laws of the State of California without regard to any conflict of laws provisions.
11. NOTICE
Any notice required or permitted by this Agreement shall be in writing and delivered by hand or sent by registered or certified mail, postage prepaid, return receipt requested, or by nationally recognized overnight delivery service, delivery charges prepaid, in each case addressed to the party to receive such notice at the address set forth below or such other address as is subsequently specified in writing in accordance with this Section. Such notice shall be deemed given or provided as of the date of such hand delivery or date of sending.
IF TO SPONSOR:
Aethlon Medical, Inc.
9635 Granite Ridge Drive, Suite 100
San Diego, CA. 92123
Attention: Jim Joyce, CEO 858-459-7800 x301
IF TO INSTITUTION:
University of California, Irvine
Office of Research, Sponsored Projects Administration
5171 California Avenue, Suite 150
Irvine, CA 92697-7600
Attention: Chris Abernethy, Principal C&G Officer, 949-824-1749
If sent via overnight delivery service to Institution, then to:
University of California, Irvine
Office of Research, Sponsored Projects Administration
5171 California Avenue, Suite 150
Irvine, CA 92617-3067
Attention: Chris Abernethy, Principal C&G Officer, 949-824-1749
| 12. | INDEMNIFICATION AND INSURANCE |
A. The Institution shall indemnify, defend, and hold harmless Sponsor and its officers, directors, employees and agents (collectively "Sponsor Indemnitees") against and from any and all claims, actions, suits, proceedings and investigations (“Claims”) arising out of, or in connection with, the Clinical Trial, but only in proportion to and to the extent that such Claims are due or claimed to be due to the negligent acts or omissions of Institution, its officers, employees or agents. Sponsor Indemnitees shall: (i) promptly notify the Institution of any Claims for which such Sponsor Indemnitees may seek indemnification hereunder, (ii) permit the Institution to conduct and exercise sole control of the defense (including all decisions relative to litigation, appeal or settlement) thereof, and (iii) fully cooperate and assist the Institution in such defense, at the Institution’s expense; provided, however, that the Institution shall not settle such Claims which admits fault or wrongdoing on the part of any of the Sponsor Indemnitees without obtaining the Sponsor Indemnitees’ prior written consent, which will not be unreasonably withheld.
| 9 |
| B. | The Sponsor shall indemnify, defend, and hold harmless the Institution, its officers, agents and employees (collectively “Institution Indemnitees”) against and from any and all claims, actions, suits, proceedings and investigations (“Claims”) arising our of, or in connection with, the Clinical Trial, but only in proportion to and to the extent that such Claims are due or claimed to be due to the negligent acts or omissions of Sponsor, its officers, directors, employees or agents. The Institution Indemnitees shall: (i) promptly notify the Sponsor of any Claim for which such Institution Indemnitee may seek indemnification hereunder, (ii) permit the Sponsor to conduct and exercise sole control of the defense (including all decisions relative to litigation, appeal or settlement) thereof, and (iii) fully cooperate and assist the Sponsor in such defense, at the Sponsor’s expense; provided, however, that the Sponsor shall not settle such Claim which admits fault or wrongdoing on the part of any of the Institution Indemnitees without obtaining the Institution Indemnitees’ prior written consent, which will not be unreasonably withheld. |
| C. | Each party certifies that it maintains a policy or program of insurance or self-insurance at levels sufficient to support the indemnification obligations assumed herein. Upon request, evidence of a party’s insurance or self-insurance shall be provided to the other party. Unless a party is self-insured, it shall name the other party as an additional insured party under its insurance policies and shall provide notice to the other party within thirty (30) days of any change in or cancellation of its coverage. |
| 13. | TERMINATION |
| A. | This Agreement may be terminated by either party for any reason upon not less than thirty (30) days prior written notice to the other. |
| B. | Within sixty (60) days of the effective date of the termination, the Institution shall return to Sponsor any funds paid by Sponsor that were not expended or irrevocably committed by the Institution prior to said date. |
| C. | Termination of this Agreement shall not affect the rights and obligations of the Parties accrued prior to the effective date of the termination. The rights and obligations under Sections 4, 6, 7, 8, 9, 11, 12, 17 and 18 shall survive the termination or expiration of this Agreement. |
14. ASSIGNMENT
Neither this Agreement nor any rights and obligations hereunder may be assigned by the Institution without the written consent of Sponsor, which will not be unreasonably withheld.
15. INDEPENDENT CONTRACTOR
The relationship of the Parties is that of independent contractors. Neither party is the partner, joint venturer, nor agent of the other and neither party has authority to make any statement, representation, commitment, or action of any kind which purports to bind the other without the other party’s prior written authorization.
16. CHANGES TO AND DEVIATIONS FROM THE PROTOCOL
| A. | If, at any time, changes in the Protocol appear desirable, such changes may be made by the Institution with the prior written consent of Sponsor. |
| B. | If, during the performance of the Clinical Trial, generally accepted standards of clinical trial studies and medical practice relating to the safety of the Subjects requires a deviation from the Protocol, such standards shall be followed. In such case, the party aware of the need for a deviation from the Protocol shall promptly inform the other party in writing of the facts necessitating such deviation. |
| 10 |
| 17. | CONFORMANCE WITH APPLICABLE LAW |
| A. | The Institution shall perform the Clinical Trial in conformance with generally accepted standards of good clinical practice, the Protocol and all applicable Federal, state and local government laws and regulations and applicable ICH guidelines governing the performance of clinical investigations, including, but not limited to, the Federal Food, Drug and Cosmetic Act and regulations of the FDA. All shipments of diagnostic specimens obtained as the result of the performance of a Clinical Trial shall comply with all applicable Federal regulations including, but not limited to, 49 CFR Part 173, such as 49 CFR 173.199 (if applicable), and the Institution shall execute any declarations required in connection therewith on forms provided, or approved, by Sponsor. |
| B. | The Institution hereby certifies that, as of the date of enrollment of each individual participating in the Clinical Trial (a “Subject”), it will obtain from each such Subject an authorization that meets the requirements of the privacy rule issued under the Health Insurance Portability and Accountability Act of 1996 ("HIPAA Privacy Rule") set forth at 45 CFR 164.508(b) and (c). Such authorization shall permit (i) all necessary uses of the individual's "protected health information", as that term is defined in the HIPAA Privacy Rule, 45 CFR 164.501, by the Institution as part of the Clinical Trial and (ii) delivery of reports that may contain some protected health information by the Institution to Sponsor and its authorized agents and the Clinical Trial team and other professionals involved in the Clinical Trial for purposes relating to the Clinical Trial or other purposes permitted by law under the randomization number assigned to the Subject. Such reports shall not contain the Subject’s name, address, telephone number, social security number or number assigned by the Institution or healthcare insurer to the Subject for the purposes of billing and locating medical records. |
| 18. | SEVERABILITY |
The invalidity or unenforceability of any term or provision of this Agreement shall not affect the validity or enforceability of any other term or provision hereof.
| 19. | WAIVER; MODIFICATION OF AGREEMENT |
No waiver, amendment, or modification of any of the terms of this Agreement shall be valid unless in writing and signed by authorized representatives of each party. Failure by either party to enforce any rights under this Agreement shall not be construed as a waiver of such rights nor shall a waiver by either party in one or more instances be construed as constituting a continuing waiver or as a waiver in other instances.
[THIS REMAINDER OF THIS PAGE IS INTENTIONALLY LEFT BLANK]
| 11 |
| 20. | ENTIRE AGREEMENT |
This Agreement represents the entire understanding of the Parties and supersedes all prior negotiations, representations or agreements, either written or oral, with respect to the subject matter hereof.
IN WITNESS WHEREOF, the Parties have executed this Agreement in duplicate by proper persons thereunto duly authorized.
| Aethlon Medical, Inc. | THE REGENTS OF THE UNIVERSITY OF CALIFORNIA | |
| By: | By: | |
| (signature) | (signature) | |
| (print or type name) | (print or type name) | |
| Title: | Title: | |
| Date: | Date: | |
| PRINCIPAL INVESTIGATOR | ||
| I have read this Agreement and understand my obligations hereunder. | ||
| By: | ||
| (signature) | ||
| (print or type name) | ||
| Title: | ||
| Date: | ||
| 12 |
EXHIBIT A
PROTOCOL
| 13 |
EXHIBIT B
PART I: PAYEE INFORMATION
| Project: | Plasma Exosome Concentration in Cancer Patients Undergoing Treatment | |
| Effective Period: | From 3/24/15 To 3/23/20 | |
| Investigator: | Edward Nelson, M.D. | |
| Institution: | University of California, Irvine | |
| Payee: | The Regents of the University of California Tax ID: 95-2226406 | |
| Mailing Address: | UCI Contracts & Grants Accounting | |
| BioSci III, Suite 1400 | ||
| Irvine, CA 92697-1050 | ||
| Attention: Rebecca Tangen, Manager/Principal Accountant | ||
PART II: PAYMENT INSTRUCTIONS AND DETAILED BUDGET
As consideration for performance under the terms of this Agreement, Sponsor agrees to pay the Institution a total sum of $178,226. Actual charges shall be based upon a rate of $3,359 per Subject. The maximum sum assumes completion of 45 Subjects in accordance to the work set forth in the Protocol. A one time, non-refundable start-up fee of $19,032.30 (which includes institutional overhead) will be made within 30 days of full execution of the Agreement. Subsequent payments will be made quarterly, within forty-five (45) days of the end of each calendar quarter, on a completed visit per subject. Invoices for the annual renewal fee shall be sent to Aethlon within two (2) months from the date of incurrence of the invoice item. Payment includes all applicable overhead. Checks will be made payable to Payee as listed above and will be sent to the address as listed under Payee.
| 14 |
Exhibit 10.2
Plasma Exosome Concentration in Cancer Patients Undergoing Treatment
Objectives of the Study:
This is a pilot study of a putative biomarker, circulating exosomes, which are increased in patients with cancer and hypothesized to play a role in tumor-associated immune suppression. We will address the following Specific Aims:
| 1) | Characterize the baseline concentration of circulation exosomes in patients with various tumor types (in and around the time of diagnosis) and the kinetics of longitudinal changes in the circulating exosome concentration associated with primary therapy (surgery, radiation therapy, chemotherapy, and/or neoadjuvant chemotherapy). |
| 2) | In patients with metastatic tumor burden, examine baseline and longitudinal changes associated with chemotherapy treatment. |
| 3) | In patients with metastatic tumor burden evaluate associations between changes in circulating exosome concentrations and response to chemotherapy. |
RESEARCH DESIGN AND METHODS
Overview of Study Design
This proposed clinical study will provide critical initial data to direct future clinical investigations of a novel treatment approach using extracorporeal hemofiltration for the removal of tumor-derived exosomes. Exosomes are small, 30-120 nm, membrane bound vesicles shed from all cells, but at higher levels from tumor cells [1], providing a logical candidate for the association between tumor burden and tumor-associated immune suppression. First described > 30 years ago, exosomes were originally thought to be cellular “garbage bags,” found in the peripheral blood, containing proteins (including various receptors and signaling molecules) and nucleic acids, (genomic, mRNA, and microRNAs) [1, 2]. Exosomes are now recognized to have many biological functions including expressing tumor associated antigens [3-7], to tumor-associated immune suppression [8-19], and have been hypothesized to directly contribute to the metastatic process and treatment resistance, but with mechanisms less well characterized [8, 12, 20-23]. It has been observed that circulating exosome concentration is increased in patients with metastatic melanoma, ovarian epithelial, prostate and colorectal neoplasms [24-29] and as such is hypothesized to vary with tumor burden.
Exosome ELLSA:
Previous studies have established that tumor- secreted exosomes display high mannose glycoprotein signatures on their surfaces [30], thus sharing this feature in common with enveloped viruses. To this end, Aethlon has examined exosomes from various tumor sources and tested their binding to GNA affinity matrices, in vitro, in studies with Dr. Douglas Taylor. In a variation of a traditional ELISA, GNA was used as a substrate for coating plates to measure exosome binding, enzyme linked lectin sorbent assay (ELLSA). Ovarian cancer exosomes that were purified by a conventional ultracentrifugation method bound to GNA and were detectable against a mannan standard curve, Figure 1.
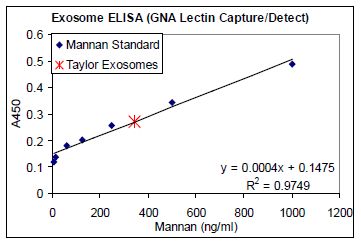
FIGURE 1. Exosome ELLSA. Binding of purified exosomes from an ovarian cancer patient (obtained by ultracentrifugation) to GNA coated plates (1 μg/mL) in a modified ELISA assay using serial dilutions of mannan and HRP labeled GNA as a detection agent to generate a standard curve.
| 15 |
Aethlon’s initial studies were conducted by Dr. Douglas Taylor using exosomes from ovarian cancer patients. Exosomes were purified from ascites fluid of two ovarian cancer patients using an established ultracentrifugation method [31, 32] or using the Hemopurifier®. The Hemopurifier® provides the capacity to elute adsorbed exosomes for subsequent evaluation. Analysis of the tumor-associated exosome marker, EpCAM, revealed that the presence of EpCAM in the material eluted from the Hemopurifier® as well as in the control exosome preparation, Figure 2 Panel A.
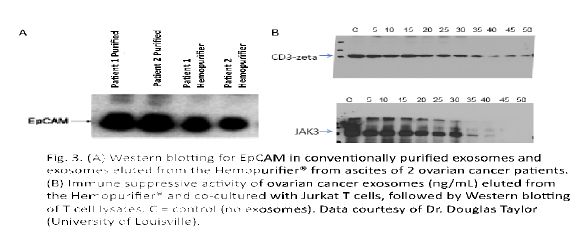
FIGURE 2. Panel A: Western blot for EpCam in conventionally purified exosomes and exosomes eluted from the Hemopurifier from ascites of 2 cancer patients. Panel B: Effect of co-culture of eluted exosomes at increasing concentrations with Jurkat T cells followed by western blotting of cell lysates for CD3 zeta chain and phospho JAK3. C=control (no exosomes).
Subjects and Recruitment:
Subject Recruitment/Informed Consent: Recruitment of participants in the study will be through the use of internal and outside referrals to the University of California, Irvine (UCI) Medical Center (UCIMC). Review and approval of this human protocol will be conducted by the UCI Institutional Review Board (IRB) Human Subjects Review Committee. Subjects must be able to understand and sign an informed consent form, which must comply with U.S. regulations (U.S. 21 CFR 50) and ICH guidelines to be eligible for this trial. All study materials, including consent forms will be provided in the patient’s primary language. In addition, each subject will be given a copy of the consent form, the Experimental Subjects’ Bill of Rights, and HIPAA Release form, all of which will be explained to the subject.
Accrual Capacity: The NCI designated Chao Family Comprehensive Cancer Center (CFCCC) at UCI has an active clinical cancer care and research program. UCI Medical Center has a large referral base and sufficient numbers of patients will be available for enrollment onto the protocol described herein. Data from the cancer registry documents that over the past three years, we have seen a steady rise from our baseline of approximately 1700 individual new “analytic” patients: 2010 = 1697, 2011 = 2000, 2012 = 2164, 2013 = 2189 (with 257 of these being new cases with metastatic disease) and are on track for a further increase in 2014. We have an outstanding record of accrual to clinical trials with over 24% of patients being accrued to clinical studies in each of these three years; the national average at NCI designated comprehensive cancer centers is 15% and nation wide is only 5%. Even given a conservative 30% accrual rate of screened and eligible patients and not accounting from recruitment from the larger surrounding non-UCI affiliated oncology care community, we have adequate capacity to successfully complete accrual to this study.
| 16 |
Study Population Demographics: Those who satisfy the inclusion/exclusion criteria and will be enrolled in the study. Patient enrollment will include all ethnic groups and both genders as available. The current demographics of the patient population seen at CFCCC and the University of California at Irvine Medical Center are as follows: 42% male; 59% female; 45%Caucasian; 5% Black; 20% Asian; 30% Hispanic.
Study Endpoints
A. Baseline circulating exosome concentration
B. Longitudinal changes in circulating exosome concentrations associated with tumor treatment.
C. Association of longitudinal changes in circulating exosome concentrations with response to treatment.
Evaluation of clinical outcome per se, (i.e., progression free or overall survival) is beyond the scope of this pilot study, we will explore potential associations with clinical outcomes and the putative biomarker, circulating exosome concentration which will provide additional pilot data to direct future studies.
Study Population - Patient Inclusion and Exclusion Criteria
Patients with a diagnosis of cancer are potential subjects for this study.
In order to be eligible for this study, patients must meet all of the following criteria:
1. Patients must have a histologically proven diagnosis of cancer.
2. Have measurable tumor burden.
3. Expected survival must be greater than six (6) months.
4. A Karnofsky Performance Status (KPS) must be 70 or greater, equivalent to ECOG 0 to ≈2 (Appendix A).
5. Patients must be >21 years of age.
6. Patients must be able to understand and sign an informed consent form, which must comply with U.S. regulations (U.S. 21 CFR 50) and ICH guidelines.
Patients with any one of the following conditions must be excluded from this trial:
1. History of repeated central line associated thrombosis or bleeding diathesis.
2. Chronic anti-coagulation therapy.
3. More than one malignant diagnosis, except for the basal cell epithelioma of the skin.
4. Persistent fever greater than 38 C.
5. Calculated CrCl less than 60 ml/min.
6. Required use of chronic corticosteroids or immune suppression for any reason including an organ allograft or HIV infection
7. Patients with any acute or chronic illness including cardiovascular disease or history of myocardial infarction, autoimmune state, or any psychiatric illness that in the opinion of the Investigators would compromise treatment.
Study Design/Sample Size:
As this is a pilot, hypothesis generating early phase non-randomized, clinical study, designed to provide initial characterization of a tumor biomarker, and is not a hypothesis testing Phase II or III study, standard calculations of “power” or “sample size” are not appropriate or applicable for this study. It is not known if there are significant differences in circulating exosome concentrations by tumor type, treatment type, or kinetics of treatment response. Thus, the final target study cohort size will be dependent upon early observations in a range of subjects from various tumor types. We will employ cohort expansion modifications as indicated. We initially plan on enrolling 5 patients with defined tumor types, see table next page, for a total initial study population of 45 subjects.
| 17 |
If analysis of the initial 5 subjects with a given tumor type suggests impact of histologic subtype (e.g. for breast cancer – hormone receptor positive, HER2/neu positive, triple negative, lobular vs. ductal), treatment type or kinetics of treatment response; expansion cohorts consisting of 5 subjects will be added, via modification of the IRB approved protocol, as needed to characterize the impact of these factors on circulating exosome concentrations.
| Tumor Type | Study Cohort |
Approximate number of cases at UCIMC on treatment with tumor present - 2013 |
| Breast adenocarcinoma | 5 | 30 |
| Colorectal | 5 | 21 |
| Gastric & Gastroesophageal | 5 | 30 |
| Pancreatic | 5 | 60 |
| Cholangiocarcinoma | 5 | 9 |
| Lung (NSCLC) | 5 | 44 |
| Head & Neck (SCC) | 5 | 42 |
| Melanoma | 5 | 22 |
| Ovarian adenocarcinoma | 5 | 23 |
Research Methodology/Study Procedures
Enrolled subjects will have standard phlebotomy performed with collection of blood into 4 ml vacutainer tubes containing K2EDTA anticoagulant. These tubes will be transported to Dr. Nelson’s laboratory at 4 C (in the presence of a cool pack) for centrifugation at 300 g (≈ 1200 RPM for tabletopcentrifuge - Beckman GH-3.8). Plasma will be removed from the vacutainer tube and distributed as 1 ml aliquots into 1.5 ml eppendorf snap top tubes labeled with the subjects 4 digit study ID number (e.g. 1001, cohort 1, subject #1). These tubes will be placed at -80 C until shipment on dry ice to Princeton NJ, for assay of exosome concentration.
Subjects will have phlebotomy performed immediately before the administration of treatment, primary treatment or treatment for advanced disease. This blood sample will be collected prior to the administration of any pre-medications for subsequent treatment, (surgery, chemotherapy, chemoradiation, or radiation alone, the latter will be rare). Each sample will be labeled to identify the patient as with any other biospecimen collected for clinical evaluation. Ideally, the first sample will be collected before any treatment has been initiated, however there is no absolute requirement that the patients have not received prior treatment if they have advanced disease. For those patients continuing treatment but with measurable, advanced disease, progression free survival and time to treatment failure will have less meaning and we hypothesize, will be less likely to demonstrate associations with circulating exosome concentrations. Never the less, the first collection will be considered the patient’s baseline value. For multiple day regimens, patients will have 4 ml of blood collected on the first and last day of the chemotherapy regimen. Additional 4 ml samples will be collected no more frequent than weekly during the first four weeks of enrollment on this protocol. Thus, regardless of whether treatment is primary (for a new diagnosis and therefore potentially primary surgical treatment), is neoadjuvant, or is secondary for advanced disease the subjects will get a minimum of 4 x 4 ml phlebotomy samples collected in the first month. Thereafter, phlebotomy will be performed with each cycle of chemotherapy (neoadjuvant or secondary) or with each post primary treatment follow-up visit for no more than 6 months from the baseline collection.
| 18 |
Endpoint Data and Evaluations:
Circulating Exosome Concentrations:
Samples of plasma shipped frozen to Princeton NJ, will be analyzed in replicate via the ELLSA assay described above, quantifying the concentration of circulating exocomes in each sample. Raw and mean data will be provided for data analyses. Grubbs test will be used to identify outliers from triplicate or larger replicate determinations of exosome concentration from each sample.
Other Data to be Collected:
Treatment and Response: Patients may be receiving so called “targeted therapies”, standard cytotoxic chemotherapy, combined chemoradiation, and/or symptomatic/palliative support without cytotoxic therapies. Select agents can induce different types of tumor cell death and may have different effects on the release of tumor-associated exosomes. It is conceivable that an immediate and profound cytotoxic effect may lead to a transient “bolus” release of tumor-associated exosomes. Thus, treatment administered will be collected to investigate the impact of particular agents or combinations thereof, on the longitudinal changes in circulating exosome concentration. Additionally, we will collect tumor response data from the clinician’s notes to assess the association between changes in tumor burden and the longitudinal changes in circulating exosome concentrations.
Progression Free Survival and Time to Relapse: All patients will be followed from the time of enrollment for PFS and TTR for a maximum of five years. These clinical parameters will be used to investigate potential associations with longitudinal changes in the circulating exosome concentration.
Sociodemographic Characteristics: Race and ethnicity will be recorded for all participants in order to ascertain whether these impact circulating exosome concentration. This will inform the design of future studies. In addition, the use of prescription and nonprescription medications will be recorded as these may also confound the circulating exosome concentration.
Cancer & Medical History: Diagnosis, prior treatment, comorbidities, as well as histology classification will be recorded. Social history including education level, activity level, will also be recorded to investigate potential confounding co-variables.
Statistical Considerations & Data Analysis
Descriptive statistics will be computed to summarize demographic and background variables, efficacy variables and toxicity variables. Comparisons of baseline characteristics will be performed using univariate analysis of variance for continuous variables and Chi-square analyses for categoric variables. Changes over time in circulating exosome concentrations and associations with other categoric variables will be evaluated by multivariate analysis of variance methods for repeated measures. Because this is an early phase study, the sample size is not based on the power necessary to test a hypotheses for a given or expected effect size.
Risk
The risk to the subjects is limited to the risk of phlebotomy and the risk of breach of HIPAA security Phlebotomy Risk: Collection of blood during the phlebotomy visit may cause pain, brief dizziness, possible fainting, slight bleeding, swelling or bruising at the collection site, and a very remote chance of infection. To minimize this risk certified and experienced phlebotomists or RNs will collect the specimens.
| 19 |
HIPAA Risk: We recognize that there is a risk for confidential information to be exposed. Biomarkers are stored in Dr. Nelson’s laboratory. Clinical data is maintained within the EMR and if transferred to any desktop computer, that computer will be maintained in a locked office, encrypted and password protected in accordance with University of California policies. Access to the computers and files is restricted by a high security system through password. Within our secure network, data will only be accessible to the study personnel who have signed confidentiality agreements, taken online IRB tutorials for the protection of human subjects and taken the Health Insurance Portability and Accountability Act online tutorial. Computers are located in offices where office doors are closed and locked after working hours and during weekends. All office doors are locked after working hours and during weekends. To additionally protect the confidentiality of participants, our central informatics system includes the following features: 1) use of encryption software to secure data transport; 2) a firewall system that protects the informatics network from intrusion or unauthorized access; 3) access to the central informatics system requires a valid password; 4) confidential data are stored in a separate secure database table and linked to data in other tables by a number only with no personal identifiers; 5) extract files and reports will not contain any confidential information.
Potential Benefits of the Proposed Research to Subjects and Others:
There will be no benefits for participants in the study.
| 20 |
References:
| 1. | Thery, C., Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep, 2011. 3: p. 15. |
| 2. | Valenti, R., et al., Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res,2 006. 66(18): p. 9290-8. |
| 3. | Schartz, N.E., et al., From the antigen-presenting cell to the antigen-presenting vesicle: the exosomes. Curr Opin Mol Ther, 2002. 4(4): p. 372-81. |
| 4. | Wolfers, J., et al., Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med, 2001. 7(3): p. 297-303. |
| 5. | Mignot, G., et al., Prospects for exosomes in immunotherapy of cancer. J Cell Mol Med, 2006. 10(2): p. 376-88. |
| 6. | Delcayre, A. and J.B. Le Pecq, Exosomes as novel therapeutic nanodevices. Curr Opin Mol Ther, 2006. 8(1): p. 31-8. |
| 7. | Hao, S., T. Moyana, and J. Xiang, Review: cancer immunotherapy by exosome-based vaccines. Cancer Biother Radiopharm, 2007. 22(5): p. 692-703. |
| 8. | Filipazzi, P., et al., Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol, 2012. 22(4): p. 342-9. |
| 9. | Whiteside, T.L., Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem Soc Trans, 2013. 41(1): p. 245-51. |
| 10. | Marton, A., et al., Melanoma cell-derived exosomes alter macrophage and dendritic cell functions in vitro. Immunol Lett, 2012. 148(1): p. 34-8. |
| 11. | Peng, P., Y. Yan, and S. Keng, Exosomes in the ascites of ovarian cancer patients: origin and effects on anti-tumor immunity. Oncol Rep, 2011. 25(3): p. 749-62. |
| 12. | Azmi, A.S., B. Bao, and F.H. Sarkar, Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev, 2013. |
| 13. | Chalmin, F., et al., Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3- dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest, 2010. 120(2): p. 457-71. |
| 14. | Xiang, X., et al., TLR2-mediated expansion of MDSCs is dependent on the source of tumor exosomes. Am J Pathol, 2010. 177(4): p. 1606-10. |
| 15. | Liu, Y., et al., Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol, 2010. 176(5): p. 2490-9. |
| 16. | Xiang, X., et al., Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer, 2009. 124(11): p. 2621-33. |
| 17. | Yu, S., et al., Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol, 2007. 178(11): p. 6867-75. |
| 18. | Clayton, A., et al., Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res, 2007. 67(15): p. 7458-66. |
| 19. | Peche, H., et al., Induction of tolerance by exosomes and short-term immunosuppression in a fully MHC-mismatched rat cardiac allograft model. Am J Transplant, 2006. 6(7): p. 1541-50. |
| 20. | Rana, S., K. Malinowska, and M. Zoller, Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia, 2013. 15(3): p. 281-95. |
| 21. | Hood, J.L., R.S. San, and S.A. Wickline, Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res, 2011. 71(11): p. 3792-801. |
| 22. | Aung, T., et al., Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci U S A, 2011. 108(37): p.15336-41. |
| 23. | Pilzer, D., et al., Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin Immunopathol, 2005. 27(3): p. 375-87. |
| 24. | Jimenez, C.R., et al., Proteomics of colorectal cancer: overview of discovery studies and identification of commonly identified cancer-associated proteins and candidate CRC serum markers. J Proteomics, 2010. 73(10): p. 1873-95. |
| 25. | Khan, S., et al., Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS One, 2012. 7(10): p. e46737. |
| 26. | Kucharzewska, P., et al., Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia- dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A, 2013. 110(18): p. 7312-7. |
| 27. | Liang, B., et al., Characterization and proteomic analysis of ovarian cancer-derived exosomes. J Proteomics, 2013. 80C: p. 171-182. |
| 28. | Logozzi, M., et al., High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One, 2009. 4(4): p. e5219. |
| 29. | Silva, J., et al., Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosomes Cancer, 2012. 51(4): p. 409-18. |
| 30. | Batista, B.S., et al., Identification of a conserved glycan signature for microvesicles. J Proteome Res, 2011. 10(10): p. 4624-33. |
| 31. | Momen-Heravi, F., et al., Current methods for the isolation of extracellular vesicles. Biol Chem, 2013. 394(10): p. 1253-62. |
| 32. | Thery, C., et al., Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol, 2006. Chapter 3: p. Unit 3 22. |
| 21 |
Exhibit 10.3
Protocol No.: UCI 14-25 Plasma Exosome Concentration in Cancer Patients Undergoing Treatment
Protocol Target Accrual: 45 PI: Edward Nelson, MD
Short Title: Plasma Exosome Concentration in Cancer Patients Undergoing Treatment
Startup Charges
| Event | Negotiated Charge |
| Protocol Set-Up & Administration Fees (Feasibility, Budget, Medicare Cost Coverage, IRB Application/Doc Prep, IRB Institutional Review Fee, Annual CRC Administrative Fees, and Study Supplies) |
15,105.00 |
| Subtotal: | 15,105.00 | |||
| Overhead Charges @ 0.0%: | 0.00 | |||
| Indirect Costs @ 26.0%: | 3,927.30 | |||
| Total Startup Charges: | 19,032.30 |
Per Subject Visit Charges (Items include 26.0% indirect charges)
| # occurances | Event | # of Subjects | Visit Reimbursement |
| 1 | Phlebotomy | 45 | 84.00 |
| 1 | Cancer Center Data Management | 45 | 1,497.00 |
| 1 | Study Oversight, Coordination, and Biostatistics | 45 | 1,778.00 |
| Total Subject Charges (Maximum) | 3,359.00 |
| Direct Cost Subtotal for (1) Subjects: | 2,665.87 | |||
| Indirect Costs @ 26.0%: | 693.13 | |||
| Total Per Subject Charges for (1) Subjects | 3,359.00 | |||
| Total Per Subject Charges for (45) Subjects | 151,155.00 |
Protocol Related Variable Passthrough Charges (Items include 26.0% indirect charges)
| Event | Negotiated Charge | |||
| Protocol Annual Renewal Prep Fee (Payment triggered at IRB renewal. Includes study project management outside visit activities for sponsor site visits, IRB renewal prep, bill reconciliation, OnCore managment, SAE reporting, IRB modifications, close out and storage, etc.) | 7,000.00 | |||
| IRB Committee Renewal Review Fee | 1,039.50 | |||
| Consent Translation (Pass through cost, based on language and length of document) | TBD | |||
| Other Tests/Procedures not included above | TBD |
| 22 |
Exhibit 99.1

Aethlon Medical Announces Cancer Clinical Trial Agreement
SAN DIEGO, April 15, 2015 /PRNewswire/ --Aethlon Medical, Inc. (OTCQB:AEMD), a pioneer in developing targeted therapeutic devices to address infectious diseases and cancer, announced today that it has entered into an investigator-initiated clinical trial agreement with the University of California, Irvine (UCI). Under the agreement, a clinical study protocol entitled, “Plasma Exosome Concentration in Cancer Patients Undergoing Treatment," will seek to enroll five individuals in each of nine defined tumor types for a total study population of up to 45 subjects. The tumor types include the following forms of cancer; Breast adenocarcinoma, Colorectal, Gastric & Gastroesophageal, Pancreatic, Cholangiocarcinoma, Lung (NSCLC), Head & Neck (SCC), Melanoma and Ovarian adenocarcinoma. The principal investigator of the study is Edward Nelson, M.D.
The study endpoints include establishing baseline exosome levels and monitoring changes in circulating exosome concentration associated with tumor treatment and the association of longitudinal changes in circulating exosome concentrations with response to treatment.
The clinical study will also provide data to help direct future clinical investigations of the Aethlon Hemopurifier® as a therapeutic candidate to reduce the presence of circulating tumor-derived exosomes, which are known to suppress the immune system of cancer patients and contribute to the spread of metastasis. Aethlon Medical believes that the Hemopurifier is a first-in-class bio-filtration device that targets the single-use removal of viruses and tumor-derived exosomes from the circulatory system.
Recruitment of participants in the study will be through the use of internal and outside referrals to the University of California, Irvine Medical Center (UCIMC). Review and approval of this human protocol will be conducted by the UCI Institutional Review Board (IRB) Human Subjects Review Committee.
About Aethlon Medical, Inc.
Aethlon Medical creates medical devices that target unmet therapeutic needs in infectious disease and cancer. The company's lead product is the Aethlon Hemopurifier®, a first-in-class device that selectively targets the rapid elimination of circulating viruses and tumor-secreted exosomes that promote cancer progression. Exosome Sciences, Inc. is a majority owned subsidiary that is advancing exosome-based products to diagnose and monitor cancer, infectious disease and neurological disorders. Additional information can be found online at www.AethlonMedical.com and connect with the Company on Twitter, LinkedIn, Facebook and Google+.
Certain statements herein may be forward-looking and involve risks and uncertainties. Such forward-looking statements involve assumptions, known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of Aethlon Medical, Inc. to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements. Such potential risks and uncertainties include, without limitation, that Exosome Sciences, Inc. will not be able to commercialize its future products, including any that can be described as a liquid biopsy, that the FDA will not approve the initiation of the Company's future clinical programs or provide market clearance of the Company's products, future human studies, whether revenue or non-revenue generating, of the Aethlon ADAPT™ system or the Aethlon Hemopurifier® as an adjunct therapy to improve patient responsiveness to established cancer or hepatitis C therapies or as a standalone cancer or hepatitis C therapy or as a broad spectrum defense against viral pathogens, including Ebola, the Company's ability to raise capital when needed, the Company's ability to complete the development of its planned products, the Company's ability to manufacture its products, either internally or through outside companies, and provide its services, the impact of government regulations, patent protection on the Company's proprietary technology, the ability of the Company to meet the milestones contemplated in the DARPA contract, product liability exposure, uncertainty of market acceptance, competition, technological change, and other risk factors. In such instances, actual results could differ materially as a result of a variety of factors, including the risks associated with the effect of changing economic conditions and other risk factors detailed in the Company's Securities and Exchange Commission filings. The Company undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
Contacts:
James A. Joyce
Chairman and CEO
(Office) 858.459.7800 x301
(Cell) 619-368-2000
jj@aethlonmedical.com
Jim Frakes
Chief Financial Officer
858.459.7800 x300
jfrakes@aethlonmedical.com
| 23 |